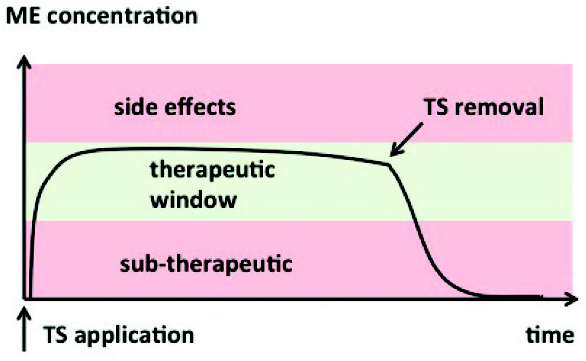
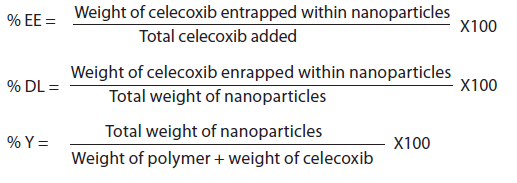
ABOUT AUTHOR:
Anilkumar
Dr.Ambedkar Institute of Technology
Bangalore, India
[email protected]
INTRODUCTION
The dissolution rate of drug from tablet is affected by its active ingredient’s surface area and consequently, affects in oral bioavailability of the product. The development of formulations containing poorly-water-soluble drugs for oral delivery can be achieved by improving their dissolution. It has been found that increasing the available surface area by reducing the particle size can often markedly improve dissolution rates and lead to dramatic improvements in bioavailability. In some cases, the decreasing drug particle through micronized powder by milling tends to agglomerate or accelerate the polymorphic conversion. According to the differences of solubility and dissolution rates of polymorphs, the bioavailability of pharmaceuticals depends on polymorphous crystals. It has been shown that the polymorph in amorphous form of drug usually dissolves more rapidly than the corresponding crystalline form. Therefore the dissolution and bioavailability of formulation containing active ingredient in amorphous form including pseudopolymorphs form such as solvates would be increased. On the other hand, the processes in making tablets, including blending, granulating, drying and especially compressing affected therapeutic property of the drug because polymorphic forms, crystal habit, size and surface area would be changed during these processes.1
REFERENCE ID: PHARMATUTOR-ART-2019
A new technique of tablet preparation was patented, a chargeable pharmaceutical tablet which could solve the mentioned problems. The tablet was prepared by loading a blank tablet with liquid form of the active pharmaceutical ingredient. In this study, candesartan displays a poorly-water-soluble drug, which results in low and erratic oral bioavailability. Attempts were made to enhance the dissolution of candesartan using “a drug-solution-dropping” technique which had success for water-soluble drug, chlorpheniramine maleate. In vitro drug dissolution rate was used as main criteria for comparison between both kinds of blank tablet with dropping drug solution and also with conventional tablet prepared by DC method.2
Therapeutic effectiveness of a drug depends upon the bioavailability and ultimately upon the solubility of drug molecules. Solubility is one of the important parameter to achieve desired concentration of drug in systemic circulation for pharmacological response to be shown. Currently only 8% of new drug candidates have both high solubility and permeability.3 The solubility of a solute is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature. In the other words the solubility can also define as the ability of one substance to form a solution with another substance. The substance to be dissolved is called as solute and the dissolving fluid in which the solute dissolve is called as solvent, which together form a solution. The process of dissolving solute into solvent is called as solution or hydration if the solvent is water.4 The transfer of molecules or ions from a solid state into solution is known as dissolution. In essence, when a drug dissolves, solid particles separate and mix molecule by molecule with the liquid and appear to become part of that liquid. Therefore, drug dissolution is the process by which drug molecules are liberated from a solid phase and enter into a solution phase. The use of poorly soluble drugs has a number of drawbacks such as increasing the dosage, administration frequency and the resultant occurrence of side effects. Furthermore, the rate-limiting step in the absorption process for poorly water-soluble drugs is the dissolution rate of such drugs in the gastro intestinal fluids rather than the rapidity of their diffusion across the gut wall; it is however, important to improve the oral bioavailability of poorly water soluble drugs by improving their dissolution rate and solubility.
TECHNIQUES OF SOLUBILITY ENHANCEMENT
There are various techniques available to improve the solubility of poorly soluble drugs. Some of the approaches to improve the solubility are 5
1) PHYSICAL MODIFICATIONS
Particle size reduction
· Micronization
· Nanosuspension
· Sonocrystalisation
· Supercritical fluid process
Modification of the crystal habit
· Polymorphs
· Pseudopolymorphs
Drug dispersion in carriers
· Eutectic mixtures
· Solid dispersions
· Solid solutions
Complexation
· Use of complexing agents
Solubilization by surfactants:
· Microemulsions
· Self microemulsifying drug delivery systems
2) CHEMICAL MODIFICATIONS
3) OTHER METHODS
· Cocrystalisation
· Cosolvency
· Hydrotrophy
· Solvent deposition
· Selective adsorption on insoluble carrier
· Use of soluble prodrug
· Functional polymer technology
· Porous microparticle technology
· Nanotechnology approaches
PHYSICAL MODIFICATIONS
Particle size reduction: Particle size reduction can be achieved by micronisation and nanosuspension. Each technique utilizes different equipments for reduction of the particle size.
Micronization: The solubility of drug is often intrinsically related to drug particle size. By reducing the particle size, the increased surface area improves the dissolution properties of the drug. Conventional methods of particle size reduction, such as communition and spray drying, rely upon mechanical stress to disaggregate the active compound. The micronisation is used to increased surface area for dissolution.4 Micronisation increases the dissolution rate of drugs through increased surface area; it does not increase equilibrium solubility. Micronization of drugs is done by milling techniques using jet mill, rotor stator colloid mills etc. Micronization is not suitable for drugs having a high dose number because it does not change the saturation solubility of the drug.6
Nanosuspension: Nanosuspensions are sub-micron colloidal dispersion of pure particles of drug, which are stabilized by surfactants. The advantages offered by nanosuspension is increased dissolution rate is due to larger surface area exposed, while absence of Ostwald ripening is due to the uniform and narrow particle size range obtained, which eliminates the concentration gradient factor. Techniques for the production of nanosuspensions include Homogenization and wet milling Active drug in the presence of surfactant is defragmented by milling. Other technique involves the spraying of a drug solution in a volatile organic solvent into a heated aqueous solution. Rapid solvent evaporation produces drug precipitation in the presence of surfactants. The nanosuspension approach has been employed for drugs including tarazepide, atovaquone, amphotericin B, paclitaxel and bupravaquone. All the formulations are in the research stage. One major concern related to particle size reduction is the eventual conversion of the high-energy polymorph to a low energy crystalline form, which may not be therapeutically active one. Drying of nanosuspensions can be done by lyophilisation or spray drying.7
Sonocrystallisation: Recrystallization of poorly soluble materials using liquid solvents and antisolvents has also been employed successfully to reduce particle size. The novel approach for particle size reduction on the basis of crystallisation by using ultrasound is Sonocrystallisation. Sonocrystallisation utilizes ultrasound power characterised by a frequency range of 20–100 kHz for inducing crystallisation. It’s not only enhances the nucleation rate but also an effective means of size reduction and controlling size distribution of the active pharmaceutical ingredients. Most applications use ultrasound in the range 20 kHz-5 MHz.8
Supercritical fluid process: A SCF exists as a single phase above its critical temperature (Tc) and pressure (Pc). SCFs have properties useful to product processing because they are intermediate between those of pure liquid and gas (i.e., liquid-like density, gas-like compressibility and viscosity and higher diffusivity than liquids). Moreover, the density, transport properties (such as viscosity and diffusivity), and other physical properties (such as dielectric constant and polarity) vary considerably with small changes in operating temperature, pressure, or both around the critical points. Hence, it is possible to fine tune a unique combination of properties necessary for a desired application. These unique processing capabilities of SCFs, long recognized and applied in the food industry, have recently been adapted to pharmaceutical applications.9
Figure 1. Typical diagram of supercritical region

Basic techniques in scf technology
Rapid Expansion of Supercritical Solutions: A supercritical solvent saturated with a solute of interest is allowed to expand at a very rapid rate, causing the precipitation of the solute. The rapid expansion/decompression is achieved by allowing into pass through a nozzle at supersonic speeds. This rapid expansion of supercritical solutions leads to super saturation of the solute in it and subsequent precipitation of solute particles with narrow particle size distributions. This process is also known as supercritical fluid nucleation (SFN). The SF is pumped through a pre-heater into the vessel containing the solid solute at a particular temperature and pressure. The SF dissolves and gets saturated with the solute, and the resultant solution is introduced into a precipitation chamber by expansion through capillary or laser-drilled nozzle. Typically, by altering the pressure, the precipitation unit is maintained at conditions where the solute has much lower solubility in the SF. During expansion or decompression phase, the density and solubilising power of the SF decreases dramatically, resulting in a high degree of solute super saturation and subsequent precipitation.10
Gas Antisolvent Recrystallisation: It is a well-known phenomenon that a poor solvent of a particular solute can be added to the solution to precipitate the solute. This is called salting out and is widely used for crystallization purposes. However, disadvantages of this technique include poor control over the precipitated crystal morphology, size distribution and presence of residual solvents.
Precipitation with Compressed Fluid Antisolvent: The solute can be crystallized from a solution using Antisolvents in two ways, Gas antisolvent recrystallisation (GAS) method or by spraying liquid into the SF antisolvent.In the latter, the antisolvent rapidly diffuses into the liquid solvent and the carrier liquid solvent a schematic view of the rapid expansion of supercritical solutions (RESS) process. The SF is pumped through a pre-heater into the vessel containing the solid solute at a particular temperature and pressure. The SF dissolves and gets saturated with the solute, and the resultant solution is introduced into a precipitation chamber by expansion through a capillary laser-drilled nozzle. Typically, by altering the pressure, the precipitation unit is maintained at conditions where the solute has much lower solubility in the SF. During expansion or decompression phase, the density and solubilising power of the SF decreases dramatically, resulting in a high degree of solute super saturation and subsequent precipitation. The morphology and size distribution of the precipitated material is a function of its preexpansion concentration and expansion conditions. The preexpansion concentration is dependent on the choice of SF, nature of solute, addition of cosolvents and operating pressure and temperature. Higher the preexpansion concentration, smaller will be the particles and narrower the particle size range.
Impregnation or infusion of polymers with bioactive materials: Some gases cause swelling of polymers or drug carriers at high pressures. This swelling behavior can be exploited for control delivery of drugs. Substances such as fragrances, pest control agents, and pharmacologically active materials can be impregnated with a solid polymer, which is exposed to a supercritical fluid during the impregnated process. The polymers evaluated in this study included polypropylene, polyethylene, ethylene-vinyl acetate copolymer, and ethylene-ethyl acrylate copolymer and causes the migration of active material in to the polymer. The diffusion of active material is increase significantly due to the swelling of polymer or drug carrier matrix when the pressure is reduced, the SCF is driven out slowly resulting in the drug loaded polymer particles it has been found that the swelling is increase with increasing temperature at a constant pressure this approach can be utilize to develop novel control release dosage form to deposit thermolabile material into the polymer.
Solution enhanced Dispersion by Supercritical Fluid: This technique was developed at the University of Bradford to overcome some of the limitations of the RESS and GAS methods. The drug solution and the SF are introduced simultaneously into the arrangement causing rapid dispersion, mixing and Extraction of the drug solution solvent by SF leading to very high super saturation ratios. The temperature and pressure together with accurate metering of flow rates of drug solution and SF through a nozzle provide uniform condition for particle formation. This helps to control the particle size of the product and by choosing an appropriate liquid solvent it is possible to manipulate the particle morphology.
Modification of the crystal habit:
Polymorphism is the ability of an element or compound to crystallize in more then one crystalline form. Different polymorphs of drugs are chemically identical, but they exhibit different physicochemical properties including solubility, melting point, density, texture and stability. Broadly polymorphs can be classified as enantiotropes and monotropes based on thermodynamic properties. In the case of an enantiotropic system, one polymorphs form can change reversibly into another at a definite transition temperature below the melting point, while no reversible transition is possible for monotropes. Once the drug has been characterized under one of this category, further study involves the detection of metastable form of crystal. Metastable forms are associated with higher energy and thus higher solubility. Similarly the amorphous form of drug is always more suited than crystalline form due to higher energy associated and increase surface area. Generally, the anhydrous form of a drug has greater solubility than the hydrates. This is because the hydrates are already in interaction with water and therefore have less energy for crystal breakup in comparison to the anhydrates for further interaction with water. On the other hand, the organic solvates have greater solubility than the nonsolvates. Some drugs can exist in amorphous form. Such drugs represent the highest energy state and can be considered as super cooled liquids. They have greater aqueous solubility than the crystalline forms because they require less energy to transfer a molecule into solvent. Thus, the order for dissolution of different solid forms of drug is Amorphous >Metastable polymorph>Stable polymorph Melting followed by a rapid cooling or recrystallization from different solvents can produce metastable forms of a drug.
Drug dispersion in carriers: The solid dispersion approach to reduce particle size and therefore increase the dissolution rate and absorption of drugs was first recognized in 1961. The term “solid dispersions” refers to the dispersion of one or more active ingredients in an inert carrier in a solid state, frequently prepared by the melting method, solvent method, or fusion solvent-method. Novel additional preparation techniques have included rapid precipitation by freeze drying and using supercritical fluids and spray drying, often in the presence of amorphous hydrophilic polymers and also using methods such as melt extrusion. The most commonly used hydrophilic carriers for solid dispersions include polyvinylpyrrolidone, polyethylene glycols, Plasdone-S630. Many times surfactants may also used in the formation of solid dispersion. Surfactants like Tween-80, Docusate sodium, and Sodium Lauryl Sulphate used. The solubility of etoposide24, glyburide, itraconazole, ampelopsin, valdecoxib, celecoxib, halofantrine can be improved by solid dispersion using suitable hydrophilic carriers. The eutectic combination of chloramphenicol/urea and sulphathiazole/ urea served as examples for the preparation of a poorly soluble drug in a highly water soluble carrier. The techniques of production of solid dispersion include Hot Melt method, Solvent Evaporation Method, Hot-melt Extrusion and Melting –solvent method.11
Complexation: Complexation is the association between two or more molecules to form a nonbonded entity with a well defined stoichiometry. Complexation relies on relatively weak forces such as London forces, hydrogen bonding and hydrophobic interactions.
Staching complexation: Staching complexes are formed by the overlap of the planar regions of aromatic molecules. Nonpolar moieties tend to be squeezed out of water by the strong hydrogen bonding interactions of water. This causes some molecules to minimize the contact with water by aggregation of their hydrocarbon moieties. This aggregation is favored by large planar nonpolar regions in the molecule. Stached complexes can be homogeneous or mixed. The former is known as self association and latter as complexation. Some compounds that are known to form staching complexes are as follows.Nicotinamid, Anthracene, Pyrene, Methylene blue, Benzoic acid, Salicylic acid, Ferulic acid, Gentisic acid, Purine, Theobromine, Caffeine, and Naphthalene. Higuchi and Kristiansen proposed a model according to which the compounds capable of undergoing stacking can be classified into two classes (classes A and B) based on their structure. The compounds in class A have higher affinity for compounds in class B than for those in class A and vice versa.12
Inclusion complexation: Inclusion complexes are formed by the insertion of the nonpolar molecule or the nonpolar region of one molecule (known as guest) into the cavity of another molecule or group of molecules (known as host). The major structural requirement for inclusion complexation is a snug fit of the guest into the cavity of host molecule. The cavity of host must be large enough to accommodate the guest and small enough to eliminate water, so that the total contact between the water and the nonpolar regions of the host and the guest is reduced. Three naturally occurring CDs are α-Cyclodextrin, β-Cyclodextrin, and γ- Cyclodextrin. The complexation with cyclodextrins is used for enhancement of solubility. Cyclodextrin inclusion is a molecular phenomenon in which usually only one guest molecule interacts with the cavity of a cyclodextrin molecule to become entrapped and form a stable association. The internal surface of cavity is hydrophobic and external is hydrophilic; this is due to the arrangement of hydroxyl group within the molecule. The kinetics of cyclodextrin inclusion complexation has been usually analyzed in terms of a one-step reaction or a consecutive two-step reaction involving intracomplex structural transformation as a second step. Cyclodextrins is to enhance aqueous solubility of drugs through inclusion complexation. It was found that cyclodextrins increased the paclitaxel solubility by 950 fold. Complex formation of rofecoxib, celecoxib, clofibrate, melarsoprol, taxol, cyclosporin A etc. with cyclodextrins improves the solubility of particular drugs.13
Approaches for Making Inclusion Complexes14
Physical blending method: A solid physical mixture of drug and CDs are prepared simply by mechanical trituration. In laboratory scale CDs and drug are mixed together thoroughly by trituration in a mortar and passes through appropriate sieve to get the desired particle size in the final product.
Kneading method: This method is based on impregnating the CDs with little amount of water or hydroalcoholic solutions to converted into a paste. The drug is then added to the above paste and kneaded for a specified time. The kneaded mixture is then dried and passed through sieve if required.
Co-precipitation technique: This method involves the co-precipitation of drug and CDs in a complex. In this method, required amount of drug is added to the solution of CDs. The system is kept under magnetic agitation with controlled process parameters and the content is protected from the light. The formed precipitate is separated by vacuum filtration and dried at room temperature in order to avoid the loss of the structure water from the inclusion complex.
Solution/solvent evaporation method: This method involves dissolving of the drug and CDs separately in to two mutually miscible solvents, mixing of both solutions to get molecular dispersion of drug and complexing agents and finally evaporating the solvent under vacuum to obtain solid powdered inclusion compound. Generally, the aqueous solution of CDs is simply added to the alcoholic solution of drugs. The resulting mixture is stirred for 24 hours and evaporated under vacuum at 45 °C. The dried mass was pulverized and passed through a 60-mesh sieve. This method is quite simple and economic both on laboratory and large scale production and is considered alternative to the spray drying technique.
Neutralization precipitation method: This method is based on the precipitation of inclusion compounds by neutralization technique and consists of dissolving the drug in alkaline solutions like sodium/ammonium hydroxide and mixing with an aqueous solution of CDs. The resultant clear solution is then neutralized under agitation using HCl solution till reaching the equivalence point. A white precipitate is being formed at this moment, corresponding to the formation of the inclusion compound. This precipitate is filtered and dried.
Milling/Co-grinding technique: A solid binary inclusion compounds can be prepared by grinding and milling of the drug and CDs with the help of mechanical devices. Drug and CDs are mixed intimately and the physical mixture is introduced in an oscillatory mill and grinded for suitable time. Alternatively, the ball milling process can also be utilized for preparation of the drug-CD binary system. The ball mill containing balls of varied size is operated at a specified speed for a predetermined time, and then it is unloaded, sieved through a 60-mesh sieve. This technique is superior to other approaches from economic as well as environmental stand point in that unlike similar methods it does not require any toxic organic solvents. This method differs from the physical mixture method where simple blending is sufficient and in co-grinding it requires to achieve extensive combined attrition and impact effect on powder blend.
Atomization/Spray drying method: Spray-drying is a common technique used in pharmaceuticals to produce a dry powder from a liquid phase. Another application is its use as a preservation method, increasing the storage stability due to the water elimination.26 This method represents one of the most employed methods to produce the inclusion complex starting from a solution. The mixture pass to a fast elimination system propitiate solvent and shows a high efficiency in forming complex. Besides, the product obtained by this method yield the particles in the controlled manner which in turn improves the dissolution rate of drug in complex form.
Lyophilization/ Freeze drying technique: In order to get a porous, amorphous powder with high degree of interaction between drug & CD, lyophilization/ freeze drying technique is considered as a suitable. In this technique, the solvent system from the solution is eliminated through a primary freezing and subsequent drying of the solution containing both drug & CD at reduced pressure. Thermolabile substances can be successfully made into complex form by this method. The limitations of this technique are long time process and yield poor flowing powdered product. Lyophilization/ freeze drying technique are considered as an alternative to solvent evaporation and involve molecular mixing of drug and carrier in a common solvent.
Microwave irradiation method: This technique involves the microwave irradiation reaction between drug and complexing agent using a microwave oven. The drug and CD in definite molar ratio are dissolved in a mixture of water and organic solvent in a specified proportion into a round bottom flask. The mixture is reacted for short time of about one to two minutes at 60°C in the microwave oven. After the reaction completes, adequate amount of solvent mixture is added to the above reaction mixture to remove the residual, uncomplexed free drug and CD. The precipitate so obtained is separated using whatman filter paper, and dried in vacuum oven at 40°C for 48 hrs.
Supercritical antisolvent technique: In this technique, carbon dioxide is used as anti-solvent for the solute but as a solvent with respect to the organic solvent. The use of supercritical carbon dioxide is advantageous as its low critical temperature and pressure makes it attractive for processing heat-labile pharmaceuticals. It is also non-toxic, nonflammable, inexpensive and is much easier to remove from the polymeric materials when the process is complete, even through small amount of carbon dioxide remains trapped inside the polymer, it poses no danger to the consumer. Supercritical particle generation processes are new and efficient route for improving bioavailability of pharmaceutically active compounds. In addition, supercritical fluid processes were recently proposed as a new alternative method for the preparation of drug cyclodextrin complexes. Supercritical carbon dioxide is suggested as a new complexation medium due to its properties of improved mass transfer and increased solvating power. This method constitutes one of the most innovators methods to prepare the inclusion complex of drug with CD in solid state. This is a non-toxic method as it is not utilizing any organic solvent, fast process, maintenance cost is low with promising results, but it requires a quite high initial cost.
In this technique, first, drug and CD are dissolved in a good solvent then the solution is fed into a pressure vessel under supercritical conditions, through a nozzle (i.e. sprayed into supercritical fluid anti-solvent). When the solution is sprayed into supercritical fluid anti-solvent, the anti-solvent rapidly diffuses into that liquid solvent as the carrier liquid solvent counter diffuses into the anti-solvent. Because of the supercritical fluid expanded solvent has lower solvent power than the pure solvent, the mixture becomes supersaturated resulting in the precipitation of the solute and the solvent is carried away with the supercritical fluid flow.
Solubilization by surfactants
Surfactants are molecules with distinct polar and nonpolar regions. Most surfactants consist of a hydrocarbon segment connected to a polar group. The polar group can be anionic, cationic, zwitterionic or nonionic. When small apolar molecules are added they can accumulate in the hydrophobic core of the micelles. This process of solubilization is very important in industrial and biological processes. The presence of surfactants may lower the surface tension and increase the solubility of the drug within an organic solvent.15
Microemulsions: The term microemulsion was first used by Jack H. Shulman in 1959. A microemulsion is a four-component system composed of external phase, internal phase, surfactant and cosurfactant. The addition of surfactant, which is predominately soluble in the internal phase unlike the cosurfactant, results in the formation of an optically clear, isotropic, thermodynamically stable emulsion. It is termed as microemulsion because of the internal or dispersed phase is < 0.1 μ droplet diameter. The formation of microemulsion is spontaneous and does not involve the input of external energy as in case of coarse emulsions. The surfactant and the cosurfactant alternate each other and form a mixed film at the interface, which contributes to the stability of the microemulsions. Non-ionic surfactants, such as Tweens and Labrafil with high hyrophile-lipophile balances are often used to ensure immediate formation of oil-in-water droplets during production. Advantages of microemulsion over coarse emulsion include its ease of preparation due to spontaneous formation, thermodynamic stability, transparent and elegant appearance, increased drug loading, enhanced penetration through the biological membranes, increased bioavailability, and less inter- and intra-individual variability in drug pharmacokinetics.16
CHEMICAL MODIFICATIONS
For organic solutes that are ionizable, changing the pH of the system may be simplest and most effective means of increasing aqueous solubility. Under the proper conditions, the solubility of an ionizable drug can increase exponentially by adjusting the pH of the solution. A drug that can be efficiently solubilized by pH control should be either weak acid with a low pKa or a weak base with a high pKa. Similar to the lack of effect of heat on the solubility of non-polar substances, there is little effect of pH on nonionizable substances. Nonionizable, hydrophobic substances can have improved solubility by changing the dielectric constant of the solvent by the use of co-solvents rather than the pH of the solvent. The use of salt forms is a well known technique to enhanced dissolution profiles. Salt formation is the most common and effective method of increasing solubility and dissolution rates of acidic and basic drugs. An alkaloid base is, generally, slightly soluble in water, but if the pH of medium is reduced by addition of acid, and the solubility of the base is increased as the pH continues to be reduced. The reason for this increase in solubility is that the base is converted to a salt, which is relatively soluble in water. The solubility of slightly soluble acid increased as the pH is increased by addition of alkali, the reason being that a salt is formed.17
OTHER METHODS
Co-crystallisation: The new approach available for the enhancement of drug solubility is through the application of the co-crystals, it is also referred as molecular complexes. If the solvent is an integral part of the network structure and forms at least two component crystal, then it may be termed as co-crystal. If the solvent does not participate directly in the network itself, as in open framework structures, then it is termed as clathrate. A co-crystal may be defined as a crystalline material that consists of two or more molecular species held together by non-covalent forces. Co-crystals are more stable, particularly as the co-crystallizing agents are solids at room temperature. Only three of the co-crystallizing agents are classified as generally recognized as safe (GRAS) it includes saccharin, nicotinamide and acetic acid limiting the pharmaceutical applications.11 Co-crystallisation between two active pharmaceutical ingredients has also been reported. This may require the use of subtherapeutic amounts of drug substances such as aspirin or acetaminophen. At least 20 have been reported to date, including caffeine and glutaric acid polymorphic co-crystals. Co-crystals can be prepared by evaporation of a heteromeric solution or by grinding the components together. Another technique for the preparation of co-crystals includes sublimation, growth from the melt, and slurry preparation. The formation of molecular complexes and co-crystals is becoming increasingly important as an alternative to salt formation, particularly for neutral compounds or those having weakly ionizable groups.18
Cosolvency: The solubilisation of drugs in co-solvents is a technique for improving the solubility of poorly soluble drug. It is well-known that the addition of an organic cosolvent to water can dramatically change the solubility of drugs. Weak electrolytes and nonpolar molecules have poor water solubility and it can be improved by altering polarity of the solvent. This can be achieved by addition of another solvent. This process is known as cosolvency. Solvent used to increase solubility known as cosolvent. Cosolvent system works by reducing the interfacial tension between the aqueous solution and hydrophobic solute. It is also commonly referred to as solvent blending. Most cosolvents have hydrogen bond donor and/or acceptor groups as well as small hydrocarbon regions. Their hydrophilic hydrogen bonding groups ensure water miscibility, while their hydrophobic hydrocarbon regions interfere with waters hydrogen bonding network, reducing the overall intermolecular attraction of water. By disrupting waters self-association, cosolvents reduce waters ability to squeeze out non-polar, hydrophobic compounds, thus increasing solubility. A different perspective is that by simply making the polar water environment more non-polar like the solute, cosolvents facilitate solubilization. Solubility enhancement as high as 500-fold is achieved using 20 % 2-pyrrolidone.19
Solubilizing agents: The solubility of poorly soluble drug can also be improved by various solubilizing materials. PEG 400 is improving the solubility of hydrochlorthiazide. Modified gum karaya (MGK), a recently developed excipient was evaluated as carrier for dissolution enhancement of poorly soluble drug, nimodipine. The aqueous solubility of the antimalarial agent halofantrine is increased by the addition of caffeine and nicotinamide.20
Hydrotrophy: Hydrotropic solubilization is one of them. Hydrotropy is a solubilization phenomenon whereby addition of large amounts of a second solute results in an increase in the aqueous solubility of another solute. Concentrated aqueous hydrotropic solutions of sodium benzoate, sodium aciculate, urea, nicotinamide, sodium citrate and sodium acetate have been observed to enhance the aqueous solubilities of many poorly water-soluble drugs. Hydrotropes are a class of amphiphilic molecules that cannot form well organized structures, such as micelles, in water but do increase the aqueous solubility of organic molecules.
Often strong synergistic effects are observed when hydrotropes are added to aqueous surfactant or polymer solutions. A hydrotrope is a compound that solubilises hydrophobic compounds in aqueous solutions. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (like surfactants) but the hydrophobic part is generally too small to cause spontaneous self aggregation. Hydrotropes do not have a critical concentration above which self-aggregation ‘suddenly’ starts to occur (as found for micelle- and vesicle-forming surfactants,which have a critical micelle concentration or CMC and a critical vesicle concentration or CVC, respectively Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilisate has been added. Hydrotropes are in use industrially. Hydrotropes are used in detergent formulations to allow more concentrated formulations of surfactants.21
Solvent Deposition: In this method, the poorly aqueous soluble drug such as nifedipine is dissolved in an organic solvent like alcohol and deposited on an inert, hydrophilic, solid matrix such as starch or microcrystalline cellulose y evaporation of solvent.
Selective Adsorption on insoluble Carriers: A highly active adsorbent such as the inorganic clays like bentonite can enhance the dissolution rate of poorly water-soluble drugs such as griseofulvin, indomethacin and prednisone by maintaining the concentration gradient at its maximum. The two reasons suggested for the rapid release of drugs from the surface of clays are– the weak physical bonding between the adsorbate and the adsorbent, and hydration and swelling of the clay in the aqueous media.
Use of soluble Prodrug: Wherein the physico-chemical properties of the drug are improved by bio-reversible chemical alteration. The most common prodrug strategy involves the incorporation of polar or ionizable moiety into the parent compound to improve aqueous solubility. The ‘post hoc pro-drug approach’ (prodrug of established drugs) has been successfully used to improve water solubility of corticosteroids, vitamins and benzodiazepines.
Functional polymer technology: Functional polymer enhances the dissolution rate of poorly soluble drugs by avoiding the lattice energy of the drug crystal, which is the main barrier to rapid dissolution in aqueous media. These polymers are ion exchange materials which contain basic or acidic groups that interact with the ionizable molecules of the surrounding medium and exchange their mobile ions of equal charge with surrounding medium reversibly and stoichiometrically. The resultant complex, known as, “Resinate”, can be formulated as a suspension, dry powder or tablet. The resins are insoluble and not absorbed into the body and the drug is released from the resinate on exposure to the physiological fluids. In other word, the dissolution rate of poorly soluble, ionizable drug like cationic, anionic and amphoteric actives can be enhanced by this technology. This can also be heat applicable to heat sensitive materials and oils.
Porous microparticle technology: In this technology, the poorly water soluble drug is embedded in a microparticle having a porous, water soluble, sponge like matrix. When mixed with water, the matrix dissolves, wetting the drug and leaving a suspension of rapidly dissolving drug particles. This is the core technology applied as HDDSTM (Hydrophobic Drug Delivery System). These drug particles provide large surface area for increased dissolution rate. The solid form has a proprietary spray drying technology that allows the size and porosity of the drug particles to be engineered as desired.
Nanotechnology approaches:
Nanotechnology will be used to improve drugs that currently have poor solubility. Nanotechnology refers broadly to the study and use of materials and structures at the nanoscale level of approximately 100 nanometers (nm) or less. For many new chemical entities of very low solubility, oral bioavailability enhancement by micronisation is not sufficient because micronized product has the tendency of agglomeration, which leads to decreased effective surface area for dissolution and the next step taken was Nanonisation.22
Nanocrystal
A nanocrystal is a crystalline material with dimensions measured in nanometers; a nanoparticle with a structure that is mostly crystalline. The nanocrystallization is defined as a way of diminishing drug particles to the size range of 1-1000 n.m.Nanocrystallization is thought to be a universal method that can be applied to any drug. There are two distinct methods used for producing nanocrystals; ’bottom-up’ and ’top-down’ development. The top-down methods (i.e. Milling and High pressure homogenization) start milling down from macroscopic level, e.g. from a powder that is micron sized. In bottom-up methods (i.e. Precipitation and Cryo-vacuum method), nanoscale materials are chemically composed from atomic and molecular components.23
Approaches for making nanocrystals
Milling: Nanoscale particles can be produced by wet-milling process. In ball mills, particle size reduction is achieved by using both impact and attrition forces. The most common models are a tumbling ball mill and a stirred media mill. One problem of this method is the degradation of mill surfaces and subsequent suspension contamination.24
High pressure homogenization: In high pressure homogenization, an aqueous dispersion of the crystalline drug particles is passed with high pressure through a narrow homogenization gap with a very high velocity. Homogenisation can be performed in water (DissoCubes) or alternatively in non-aqueous media or water-reduced media (Nanopure). The particles are disintegrated by cavitation and shear forces. The static pressure exerted on the liquid causes the liquid to boil forming gas bubbles. When exiting from the gap, gas bubbles collapse under normal air pressure. This produces shock waves which make the crystals collide, leading to particle disintegration. A heat exchanger should be used when operating on temperature sensitive materials because high pressure homogenization causes increase in the sample temperature. The particle size obtained during the homogenization process depends primarily on the nature of the drug, the pressure applied and the number of homogenization cycles.25
Precipitation: In the precipitation method a dilute solution is first produced by dissolving the substance in a solvent where its dissolution is good. The solution with the drug is then injected into water, which acts as a bad solvent. At the time of injection, the water has to be stirred efficiently so that the substance will precipitate as nanocrystals. Nanocrystals can be removed from the solution by filtering and then dried in air.
Cryo-vacuum method: In this method the active ingredient to be nanonized is first dissolved in water to attain a quasi-saturated solution. The method is based on sudden cooling of a solvent by immersing the solution in liquid nitrogen (-196 °C). Rapid cooling causes a very fast rise in the degree of saturation based on the decrease of solubility and development of ice crystals when the temperature drops below 0°C. This leads to a fast nucleation of the dissolved substance at the edges of the ice crystals. The solvent must be completely frozen before the vessel is removed from the liquid nitrogen. Next the solvent is removed by sublimation in a lyophilization chamber where the temperature is kept at constant -22°C and the pressure is lowered to 10-2 m.bar. Cryo-assisted sublimation makes it possible to remove the solvent without changing the size and habit of the particles produced, so they will remain crystalline. The method yields very pure nanocrystals since there is no need to use surfactants or harmful reagents.26
NanoMorph: The NanoMorph technology is to convert drug substances with low water-solubility from a coarse crystalline state into amorphous nanoparticles. A suspension of drug substance in solvent is fed into a chamber, where it is rapidly mixed with another solvent. Immediately the drug substance suspension is converted into a true molecular solution. The admixture of an aqueous solution of a polymer induces precipitation of the drug substance. The polymer keeps the drug substance particles in their nanoparticulate state and prevents them from aggregation or growth. Water redispersable dry powders can be obtained from the nanosized dispersion by conventional methods, e.g. spray-drying. Using this technology the coarse crystalline drug substances are transformed into a nanodispersed amorphous state, without any physical milling or grinding procedures. It leads to the preparation of amorphous nanoparticles.27
Dissocubes: Dissocubes technology is based on piston–gap high-pressure homogenization. The main advantages of this technology are ease of scale-up, little batch-to-batch variation, and aseptic production for parenteral administration.
Nanocrystal technology: Nanocrystal technology can be used to formulate and improve compound activity and final product characteristics of poorly water-soluble compounds. The nanocrystal technology can be incorporated into all parenteral and oral dosage forms, including solid, liquid, fast-melt, pulsed-release, and controlled-release dosage forms.
Nanoedge technology: Nanoedge technology is a formulation toolbox for poorly water-soluble drugs. It is a useful technology for active ingredients that have high melting points and high octanol-water partition coefficients. It is based on direct homogenization, microprecipitation, and lipid emulsions.
Nanopure technology: In Nanopure technology, poorly water-soluble drugs are transferred to drug nanocrystals via a high-pressure homogenization process. The drug powder is dispersed in a surfactant solution and the forces in the high-pressure homogenizer are strong enough to disintegrate the coarse drug powder into drug nanoparticles with a mean diameter, typically between 200–600 nm.
Crititech technology: Crititech Technology is based on PCA. Crititech uses ultrasonic energy produced by a converging–diverging nozzle or an electromechanical oscillator to shatter droplets into even droplets. This technique alone would not cause submicron particles to form because the droplets tend to coalesce immediately into larger drops. In the crititech procedure, the drug-laden solvent is sprayed into a flowing stream of supercritical carbon dioxide, which allows for a rapid mass transfer of solvent into the stream of supercritical carbon dioxide. This rapid mass transfer forces precipitation or crystallization to occur before the coalescence of droplets. The ultrasonic nozzle-based process is capable of producing discrete nanoparticles in a narrow size range. Moreover, crititech’s proprietary particle-harvesting device allows continuous processing of compounds in closed systems with complete recovery of solvents and carbon dioxide for reuse or safe disposal.
Nanocochleate Technology: Nanocochleate delivery vehicles (also known as bioral technology) are a broad-based enabling technology for the delivery of many therapeutic products. These molecules are stable phospholipid-cation precipitates composed of simple, naturally occurring materials such as phosphatidylserine and calcium. They consist of alternating layers of phospholipid and multivalent cations existing as stacked sheets, or continuous, solid, lipid bilayer sheets rolled up in a spiral configuration, with little or no internal aqueous space. Unique properties of nanocochleates have been used to mediate and enhance the oral bioavailability of a broad spectrum of important but difficult-to-formulate biopharmaceuticals, including compounds with poor water solubility, protein and peptide drugs, and large hydrophilic molecules. Nanocochleate formulations are widely suitable to a broad range of therapeutic applications which include the oral delivery of amphotericin B (bioral amphotericin B), large DNA constructs and plasmids (bioral DNA vaccines and bioral gene therapy), peptide formulations, anti-inflammatory formulations (bioral aspirin) and peptide-based vaccines. Controlled-flow cavitation (CFC) technology. CFC can be used to develop advanced materials for emerging applications and to design processes that enhance existing products and processes. CFC technology is based on hydrodynamic cavitation, which involves the formation, growth, and implosive collapse of vapor bubbles in a liquid created by fluctuations in fluid pressure. In this process, the formation, size, density, speed of collapse, intensity of implosion and other energetics of cavitation bubble creation, and collapse are controlled to produce the necessary energy dissipation levels and desired effects on the process medium.
Biopharmaceutical classification system
Introduction
The Biopharmaceutics Classification System (BCS) is a drug development tool that allows estimation of the contributions of three major factors, dissolution, solubility, and intestinal permeability, that effect oral drug absorption from immediate release (IR) solid oral products. It was first introduced into regulatory decision-making process in the guidance document on Immediate Release Solid Oral Dosage Forms: Scale-Up and Post Approval Changes. A recent draft guidance document entitled “Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Containing Certain Active Moieties/Active Ingredients Based on a Biopharmaceutics Classification System” proposes to further expand the regulatory applications of BCS and also recommends methods for classifying drugs and IR drug products.
In Vitro Dissolution Tests and Bioequivalence Assessment:
Need for BCS
The most widely applied dissolution test methods for IR products are based on the USP’s apparatus I (basket) or II (paddle) at agitation rates of 100 and 50 rpm, respectively. Typically 900 ml of an aqueous dissolution media are used. Historical data suggests that in vitro dissolution methods are generally sensitive to formulation factors that affect drug dissolution process and often it is observed that two products that exhibit some dissolution differences, in vitro, may provide similar drug concentration time profiles in blood. These observations suggest that for many IR products, dissolution in vivo may not be the rate-limiting process. The observed variability in blood level profiles may be due to variability in the physiological processes and not due to minor dissolution differences in products being tested. In other instances significant differences have been observed in the blood level profiles of two products that meet the “one-point” acceptance criteria outlined in an application or compendial dissolution specification. On rare occasions an inverse in vitro – in vivo relationship, i.e., higher peak drug concentration in blood for a product that exhibits a relatively slow rate of dissolution in vitro, in a particular media, compared with another product, have also been observed. Such examples of “failure” of in vitro dissolution tests to signal bio-in-equivalence have hindered the use of dissolution tests for assessing bioequivalence between two pharmaceutically equivalent products. Comprehensive research studies designed to elucidate mechanistic reasons for such failures are generally not available in the public domain. Also, data from such failed studies are generally not submitted to the Agency.
Possible causes for such differences may include; 1) inappropriate specification (dissolution test conditions, primarily media composition, and acceptance criteria), (2) presence of an excipient that may alter drug absorption, and (3) other reasons (for example, statistical type II error).
Experience gained through development of traditional in vitro – in vivo correlations (e.g., Level A, B, or C correlations) for IR products containing poorly soluble drugs and for extended release products suggests a significant degree of formulation dependency or specificity associated with such correlations. Therefore, for products that are likely to exhibit slow in vivo dissolution, in vitro – in vivo correlations need to be established and their predictive performance verified through experimentation. Future research in this area should address how to a priori identify dissolution test conditions that yield robust in vitro – in vivo correlations that are applicable to a wide range of formulations.
If the regulatory utility of dissolution tests for IR products are to be expanded, their reliability must be improved. For IR products this may be achieved by considering the mechanistic relationships between drug dissolution, physico-chemical characteristics of drugs, gastrointestinal physiology and absorption or permeation processes. To this effect BCS provides, with minimal reliance on in vivo pharmacokinetic data, a rational mechanistic frame work for developing reliable dissolution tests for assessing bioequivalence. The BCS also provides a means for identifying when dissolution in vivo is likely or not likely to be rate- limiting and allows for managing risks associated with reliance on in vitro dissolution for bioequivalence assessment.
The Biopharmaceutics Classification System: Class Boundaries

Figure2 : biopharmaceutical classification system
In the BCS, a drug is classified as belonging to 1) high or low solubility class, 2) high or low permeability class, and 3) an IR dosage form is categorized as belonging to a rapid or slow dissolving class. The solubility class boundary is based on the highest dose strength of an IR product that is the subject of a bioequivalence assessment. A drug substance is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over the pH range of 1-8. The volume estimate of 250 ml is derived from typical bioequivalence study protocols that prescribe administration of a drug product to fasting human volunteers with a glass (about 8 ounces) of water. This boundary value is a reflection of the minimum fluid volume anticipated in the stomach at the time of drug administration during a typical fasting bioequivalence study.
The permeability class boundary is intended to identify drugs that exhibit complete absorption such as Class I drugs. When administered as a solution, highly permeable drugs would exhibit complete absorption. Rapidly dissolving IR products of a highly soluble and highly permeable drugs are expected to exhibit complete absorption. The high permeability class membership may be documented when: 1) the extent of absorption in humans is greater than 90% (of an administered dose or that of an intravenous reference dose) and the drug is determined to be stable in the gastrointestinal tract, or 2) intestinal permeability is determined to be high compared with selected reference compounds in well characterized experimental methods such as, in vivo (e.g., intestinal perfusion in humans), in situ (e.g., intestinal perfusion in animals), or in vitro (e.g., Caco-2 cell cultures) tests and the drug is determined to be stable in the gastrointestinal tract.
SUPERDISINTEGRANTS
Disintegrants are agents added to tablet and some encapsulated formulations to promote the breakup of the tablet and capsule “slugs’ into smaller fragments in an aqueous environment there by increasing the available surface area and promoting a more rapid release of the drug substance. They promote moisture penetration and dispersion of the tablet matrix. Tablet disintegration has received considerable attention as an essential step in obtaining fast drug release. The emphasis on the availability of drug highlights the importance of the relatively rapid disintegration of a tablet as a criterion for ensuring uninhibited drug dissolution behavior. Number of factors affects the disintegration behavior of tablets.
The disintegrants have the major function to oppose the efficiency of the tablet binder and the physical forces that act under compression to form the tablet. The stronger the binder, the more effective must be the disintegrating agents in order for the tablet to release its medication. Ideally, it should cause the tablet to disrupt, not only into the granules from which it was compressed, but also into powder particles from which the granulation was prepared. Disintegrants are an essential component to tablet formulations. The ability to interact strongly with water is essential to disintegrant function. Combinations of swelling and/or wicking and/or deformation are the mechanisms of disintegrant action. A disintegrant used in granulated formulation processes can be more effective if used both “intragranularly” and “extragranularly” thereby acting to break the tablet up into granules and having the granules further disintegrate to release the drug substance into solution. However, the portion of disintegrant added intragranularly (in wet granulation processes) is usually not as effective as that added extragranularly due to the fact that it is exposed to wetting and drying (as part of the granulation process) which reduces the activity of the disintegrant. Since a compaction process does not involve its exposure to wetting and drying, the disintegrant used intragranularly tends to retain good disintegration activity. There are three methods of incorporating disintegrating agents into the tablet: A. Internal Addition (Intragranular) B.External Addition (Extragranular) C. Partly Internal and External. In a direct compression process, drug is blended with a variety of excipients, subsequently lubricated and directly compressed into a tablet. A disintegrant used in this type of formulation, simply has to break the tablet apart to expose the drug substance for dissolution.
Most common tablets are those intended to be swallowed whole and to disintegrate and release their medicaments rapidly in the gastrointestinal tract (GIT). The proper choice of disintegrant and its consistency of performance are of critical importance to the formulation development of such tablets. In more recent years, increasing attention has been paid to formulating not only fast dissolving and/or disintegrating tablets that are swallowed, but also orally disintegrating tablets that are intended to dissolve and/or disintegrate rapidly in the mouth. Most prior studies have focused on the functionrelated properties of superdisintegrants with special emphasis on correlating these functional properties to disintegrant efficiency and drug release rate. Water penetration rate and rate of disintegration force development are generally positively related to disintegrant efficiency in nonsoluble matrices. However, such a positive correlation is not always observed between tablet disintegration time and drug dissolution rate.
MECHANISM OF TABLET DISINTEGRATION
A. Swelling: Although not all effective disintegrants swell in contact with water, swelling is believed to be a mechanism in which certain disintegrating agents (such as starch) impart the disintegrating effect. By swelling in contact with water, the adhesiveness of other ingredients in a tablet is overcome causing the tablet to fall apart.
B. Porosity and Capillary Action (Wicking): Effective disintegrants that do not swell are believed to impart their disintegrating action through porosity and capillary action. Tablet porosity provides pathways for the penetration of fluid into tablets. The disintegrant particles (with low cohesiveness & compressibility) themselves act to enhance porosity and provide these pathways into the tablet. Liquid is drawn up or “wicked” into these pathways through capillary action and rupture the interparticulate bonds causing the tablet to break apart.

C. Deformation: Starch grains are generally thought to be “elastic” in nature meaning that grains that are deformed under pressure will return to their original shape when that pressure is removed. But, with the compression forces involved in tableting, these grains are believed to be deformed more permanently and are said to be “energy rich” with this energy being released upon exposure to water. In other words, the ability for starch to swell is higher in “energy rich” starch grains than it is for starch grains that have not been deformed under pressure. It is believed that no single mechanism is responsible for the action of most disintegrants. But rather, it is more likely the result of inter-relationships between these major mechanisms.
D. Due to disintegrating particle/particle repulsive forces: Another mechanism of disintegration attempts toexplain the swelling of tablet made with ‘nonswellable’disintegrants. Guyot-Hermann has proposed a particlerepulsion theory based on the observation thatnonswelling particle also cause disintegration of tablets.The electric repulsive forces between particles are themechanism of disintegration and water is required for it.Researchers found that repulsion is secondary towicking.

In recent years, several newer agents have been developed known as “Superdisintegrants”. These newer substances are more effective at lower concentrationswith greater disintegrating efficiency and mechanicalstrength. On contact with water the superdisintegrantsswell, hydrate, change volume or form and produce adisruptive change in the tablet. Effectivesuperdisintegrants provide improved compressibility, compatibility and have no negative impact on themechanical strength of formulations containing high-dosedrugs. Super disintegrants offer significant improvementsover starch. But hygroscopicity may be a problem in someformulations. As day’s passes, demand for fasterdisintegrating formulation is increased. So, pharmacistneeds to formulate disintegrants i.e. Superdisintegrantswhich are effective at low concentration and have greater disintegrating efficiency and they are more effective intragranularly. And this superdisintegrants act by swelling and due to swelling pressure exerted in the outer direction or radial direction, it causes tablet to burst or the accelerated absorption of water leading to an enormous increase in the volume of granules to promote disintegration. Three major groups of compounds have been developed which swell to many times their original size when placed in water while producing minimal viscosity effects. Different commonly used superdisintagrants are
1. Modified Starches- Sodium Carboxymethyl Starch (Sodium Starch Glycolate)
It is possible to synthesize sodium starch glycolate from a wide range of native starches, but in practice potato starch is used as it gives the product with the best disintegrating properties. After selection of the appropriate starch source the second step is the crosslinking of the potato starch. This is typically carried out using an FDA approved starch esterifying agent such as sodium trimetaphosphate or phosphorus oxychloride in alkaline suspension. The effect of introduction of the large hydrophilic carboxymethyl groups is to disrupt the hydrogen bonding within the polymer structure. This allows water to penetrate the molecule and the polymer becomes cold water soluble. The effect of the crosslinking is to reduce both the water soluble fraction of the polymer and the viscosity of dispersion in water. The optimum balance between the degree of substitution and the extent of cross-linking allows for rapid water uptake by the polymer without the formation of a viscous gel that might impede dissolution.
2. Cross-linked polyvinylpyrrolidone (crospovidone)
Crospovidone quickly wicks saliva into the tablet to generate the volume expansion and hydrostatic pressures necessary to provide rapid disintegration in the mouth. Unlike other superdisintegrants, which rely principally on swelling for disintegration, Crospovidone superdisintegrants use a combination of swelling and wicking. When examined under a scanning electron microscope, crospovidone particles appear granular and highly porous. This unique, porous particle morphology facilitates wicking of liquid into the tablet and particles to generate rapid disintegration. Due to its high crosslink density, crospovidone swells rapidly in water without gelling. Other superdisintegrants have a lower crosslink density and, as a result, form gels when fully hydrated, particularly at the higher use levels in ODT formulations. Unlike other superdisintegrants which are either poorly compressible or non-compressible, Crospovidone disintegrants are highly compressible materials as a result of their unique particle morphology. In contrast to sodium starch glycolate and croscarmellose sodium, Crospovidone superdisintegrants exhibit virtually no tendency toward gel formation, even at high use levels. Disintegrants that gel can result in ODT and chewable products with an unpleasant, gummy texture.
Crospovidone superdisintegrants provide the best overall sensory experience as well as rapid disintegration and robust tablets.
3. Modified Cellulose (croscarmellose sodium) Croscarmellose sodium is described as a cross-linked polymer of carboxymethylcellulose. Apart from the differences between the starch and cellulose polymer backbones, there are Differences between the synthetic processes used to modify the polymer. Most importantly, the DS of croscarmellose sodium is higher than that of sodium starch glycolate, and the mechanism of crosslinking is different. The substitution is performed using Williamson’s ether synthesis to give the sodium salt of carboxymethylcellulose. A key difference from the chemistry of SSG is that some of the carboxymethyl groups themselves are used to cross-link the cellulose chains, the process being accomplished by dehydration. Thus the cross-links are carboxyl ester links rather than phosphate ester links as in Primojel.

4. Soy polysaccharide- It is a natural super disintegrant that does not contain any starch or sugar so can be used in nutritional products.
5. Cross-linked alginic acid – It is insoluble in water and disintegrates by swelling or wicking action. It is a hydrophilic colloidal substance, which has high sorption capacity. It is also available as salts of sodium and potassium.
6. Gellan gum – It is an anionic polysaccharide of linear tetrasaccharides, derived from Pseudomonas elodea having good superdisintegrant property similar to the modified starch and celluloses.
7. Xanthan gum – Xanthan Gum derived from Xanthomonas campestris is official in USP with high hydrophilicity and low gelling tendency. It has low water solubility and extensive swelling properties for faster disintegration.
8. Calcium Silicate – It is a highly porous, lightweight superdisintegrant, which acts by wicking action.
Ion exchange resins – The INDION 414 has been used as a superdisintegrant for ODT. It is chemically cross-linked polyacrylic, with a functional group of – COO – and the standard ionic form is K+. It has a high water uptake capacity. It is a high purity pharmaceutical grade weak acid cation exchange resin supplied as a dry powder. It is an extremely effective tablet disintegrant which provides the necessary hardness and chemical stability to the tablet. The product swells up to a very great extend when in contact with water or gastrointestinal fluids causing rapid disintegration without the formation of lumps. It is a high molecular weight polymer, therefore it is not absorbed by the human tissues and totally safe for human consumption. It has several advantages.
Advantages
- Remarkable tendency on wetting causing rapid disintegration
- No lump formation on disintegration
- Compatible with commonly used therapeutical agents and excipients.
- Work equally effective in hydrophilic and hydrophobic formulations.
- Provides good mechanical strength to the tablet facilitating easy packing and transportation.
- Does not stick to the punches and dyes.
Although there are many superdisintegrants, which show superior disintegration, the search for newer disintegrants is ongoing and researchers are experimenting with modified natural products, like formalin casein, chitin, chitosan, polymerized agar acrylamide, xylan, smecta, key jo-clay, crosslinked carboxymethyl guar and modified tapioca starch. Studies have suggested that the water insoluble superdisintegrants show better disintegration property than the slightly water soluble agents, since they do not have a tendency to swell. Superdisintegrants that tend to swell show slight retardation of the disintegration property due to formation of viscous barrier. There is no particular upper limit regarding the amount of superdisintegrant as long as the mechanical properties of the tablet are compatible with its intended use. The superdisintegrant may be used alone or in combination with other superdisintegrants. Commercially available superdisintegrants are listed in the table given below.
Table :01 Various Superdisintegrants and Their Properties
| Superdisintegrants | Commercially available grades | Mechanism of action | Special comment |
| Crosslinkedcellulose | Crosscarmellose®Ac-Di-Sol®, Nymce ZSX®Primellose®, Solutab®,Vivasol®, L-HPC. | Swells 4-8 folds in < 10 seconds.Swelling and wicking both. | Swells in two dimensions. Direct compression or Granulation Starch free. |
| Crosslinked PVP | Crosspovidon M®Kollidon®Polyplasdone® | Swells very little and returns to original size after compression but act by capillary action. | Water insoluble and spongy in nature so get porous tablet. |
| Crosslinked starch | Explotab®Primogel® | Swells 7-12 folds in < 30 seconds. | Swells in three dimensions and high level serve as sustain release matrix. |
| Crosslinked alginicacid | Alginic acid NF | Rapid swelling in aqueous medium or wicking action. | Promote disintegration in both dry or wet granulation. |
| Soy polysaccharides | Emcosoy® | Does not contain any starch or Sugar. Used in nutritional products. | |
| Calcium silicate | Wicking action. | Highly porous, Light weight, |
REVIEW OF LITERATURE
Orawan Chitvanich et al., introduced the method toimprove dissolution rate of a poorly water soluble drug from tablet by drug-solution-dropping technique. Diazepam was used as a model drug. Absolute alcohol and dichloromethane were used to prepare diazepam solution. The 50 μl solution (5 mg diazepam) was dropped on blank tablet by using microsyringe. Two kinds of blank tablets were prepared by direct compression (DC) and wet granulation (WG) methods, using dicalcium phosphate dihydrate and lactose as diluents, respectively, with 1000, 1400 or 1800 kg compression force. The surfaces of diazepam-solution-dropping tablets were characterized by Scanning electron microscope (SEM). Their morphologies revealed the smoother surface than that of blank tablet, particularly from wet granulation method of higher compression force but not being clear to point out diazepam particles on the surface. X-ray diffraction by monochromator (single crystal) mode was also used to point out the crystalline or amorphous form of the drug. X-ray monochromator analysis could not be used to confirm the crystallinity of diazepam on the surface of prepared tablet. Differential scanning calorimetry thermatogram showed the peak of diazepam only in the tablet prepared from wet granulaion blank tablet. Dissolution profiles of the prepared tablet from the two kinds of blank tablets were compared to diazepam tablet prepared by the conventional direct compression and wet granulation technique. The result profiles revealed that this drug-solution-dropping technique could be applied especially to poorly-water-soluble drug such as diazepam by using blank tablet at 1000 kg compression force. The dissolution rate of drug-solution-dropping tablet (DSDT) from blank tablet prepared by WG method was faster than that of DSDT from blank tablet prepared by DC method. Certainly, more uniformity of active ingredient is also an advantage of the drug-solution-dropping tablet prepared.
Orawan Chitvanich et al., study is an attempt to design a novel method, drug-solution-dropping, in preparing tablets expected to release the drug faster than from conventional method. Chlorpheniramine maleate (CPM) was used as a model drug. Firstly, we prepared tablets containing no drug (blank tablets) by direct compression (DC) with dicalcium phosphate dihydrate, croscarmellose sodium and magnesium stearate as filler, super disintegrant and lubricant, respectively. Another kind of blank tablet was prepared by wet granulation (WG), using lactose as diluent. Absolute alcohol and dichloromethane were used to prepare CPM solution in a concentration of 100 micrograms per microliter. The prepared solution of 40 microliters (4 mg CPM) was dropped on each blank tablet by using microsyringe. A scanning electron microscope (SEM) was used to characterize both blank and CPM-solution-dropping tablet. Their morphology showed that the particle size of the dropped CPM in the tablet was reduced. X-ray monochromator (single crystal) analysis and differential scanning calorimetry (DSC) were used to examine the crystalline of CPM after dropping onto both kinds of blank tablets. Both techniques could not characterize powder or granule which was taken from the drug-solution-dropping tablet whether it was the drug particle or the excipients. Dissolution profiles of the CPM-solution-dropping tablet from DC and WG blank tablets were compared to CPM tablets prepared by conventional DC and WG method and also to commercial tablets. The results of the dissolution tests revealed that the drug-solution-dropping tablet could be considered a novel form to promote a faster drug release rate especially the drug-solution-dropping prepared from DC blank tablet with 1000 kg of compression force and WG blank tablet with 1800 kg of compression force. Having more uniformity of content is also an advantage of the drug-solution-dropping tablet prepared.
Mohanachandran et al., studied the enhancement of solubility, dissolution rate and bioavailability of drug is a very challenging task in drug development, nearly 40% of the new chemical entities currently being discovered are poorly water soluble drugs. Aqueous solubility of any therapeutically active substance is a key property as it governs dissolution, absorption and thus the in vivo efficacy. Orally administered drugs completely absorb only when they show fair solubility in gastric medium and such drugs shows good bioavailability. The solubility and dissolution properties of drugs play an important role in the process of formulation development. Problem of solubility is a major challenge for formulation scientist which can be solved by different technological approaches during the pharmaceutical product development work. The present review deals in detail about the different techniques used for the improvement of the solubility and dissolution rate of poorly water soluble drugs.
Anuj Kumar et al., Among all newly discovered chemical entities about 40% drugs are lipophillic and fail to reach market due to their poor aqueous solubility. For orally administered drugs solubility is one of the rate limiting parameter to achieve their desired concentration in systemic circulation for pharmacological response. Problem of solubility is a major challenge for formulation scientist, which can be solved by different technological approaches during the pharmaceutical product development. Solid dispersion, Micronization, Salt formation, are some of the vital approaches routinely employed to enhance the solubility of poorly soluble drugs but each approach has some limitation and advantages. Novel techniques like Nano-suspension, Supercritical processing, Cryogenic technology may allow greater opportunities in the delivery of poorly soluble drugs. The solubility behavior of drugs remains one of the most challenging aspects in formulation development. The present review is devoted to various traditional and novel techniques for enhancing drug solubility to reduce the percentage of poorly soluble drug candidates eliminated from the development.
Tansel C et al., prepared fast-disintegrating tablets of diclofenac potassium with sufficiently integrity as well as a pleasant taste using two different fillers and binders. Tablets were made with direct compression method. Porosity, hardness and disintegration time were determined. It carried out using a validated spectrophotometric method for the analysis of drug. Fast disintegration tablet of diclofenac potassium with durable structure and desirable taste can be prepared using both fillers and binders. Single dose of fast disintegration tablet was effective in relieving the pain.
Narmada GYet al., formulated fast dissolving tablet of amlodipine besylate for rapid action. Direct compresion method was adapted to prepare the tablets by using a 23full factorial design. FT-IR and D.T.A studies revealed that there was no physico-chemical interaction between amlodipine besylate and other excipients. All formulations are evaluated for pre-compression and post-compression parameters, wetting time, water absorption ratio. The results obtained showed that the quantity of starch potato, sodium starch glycolate, camphor significantly affect response variables. The results indicate that the optimized tablet formulation provides a short DT of 8 sec with sufficient crushing strength and acceptable friability. Stability studies of optimized formulation revealed that formulation is stable.
Kevin GCet al., prepared fast disintegrating tablets of Aceclofenac using different super disintegrants following direct compresionmethod. The sodium starch glycolate, croscarmellose sodium and pregelatinized starch (Starch 1500) were used in different concentrations according to the simplex lattice design as the super disintegrants, and the tablets were evaluated for diameter, thickness, hardness, friability, weight variation, wetting time, percentage of water absorption, disintegration time and in vitro dissolution studies. The result showed the disintegration time of all formulation showed less than 89 seconds. Formulation containing equal amount of Croscarmellose sodium and pregelatinized starch showed fastest disintegration than other formulations containing Starch 1500, cros carmellose sodium and sodium starch glycolate in various proportions and the percentage drug release was 99.5 within 10 minutes.
Kethan Tet al., studied the Solubility, the phenomenon of dissolution of solute in solvent to give a homogenous system, is one of the important parameters to achieve desired concentration of drug in systemic circulation for desired (anticipated) pharmacological response. Low aqueous solubility is the major problem encountered with formulation development of new chemical entities as well as for the generic development. More than 40% NCEs (new chemical entities) developed in pharmaceutical industry are practically insoluble in water. Solubility is a major challenge for formulation scientist. Any drug to be absorbed must be present in the form of solution at the site of absorption. Various techniques are used for the enhancement of the solubility of poorly soluble drugs which include physical and chemical modifications of drug and other methods like particle size reduction, crystal engineering, salt formation, and solid dispersion, use of surfactant, complexation, and so forth. Selection of solubility improving method depends on drug property, site of absorption, and required dosage form characteristics.
AIM & OBJECTIVE
AIM:
The aim of the study is to improve dissolution rate of a poorly water soluble drug from tablet by drug-solution-dropped technique. Candesartan was used as a model drug.
OBJECTIVE:
The main objectives are as follows:
* To develop the formulations containing poorly-water-soluble drugs for oral delivery by improving their dissolution and bio availability.
* To study the effect of polymorphism on the solubility of active ingredient.
* To perform the compatibility studies of drug with excipients.
* To prepare tablets with combination of superdisintegrants employing different ratios and to select best optimized formula.
* To study the morphology of the prepared tablet by using SEM photography.
PLAN OF WORK
* LITERATURE SURVEY
* SELECTION OF DRUG AND EXCIPIENTS
* PROCUREMENT OF DRUG AND EXCIPIENTS
* EXPERIMENTAL WORK
– PREFORMULATION STUDY
* IDENTIFICATION OF DRUG
• ORGANOLEPTIC PROPERTIES
• DETERMINATION OF MELTING POINT
• SOLUBILITY STUDY
• UV SPECTROPHOTOMETRIC STUDY
• PREPARATION OF STANDARD CURVE
* DRUG – EXCIPIENTS COMPATIBILITY STUDY
• INFRA-RED SPECTROSCOPY (IR) STUDY
* FORMULATION OF DRUG SOLUTION DROPPED TABLETS AND OPTIMIZATION
* EVALUATION
* EVALUATION OF POWDER BLENDS:-
– ANGLE OF REPOSE
– BULK DENSITY
– TAPPED DENSITY
– CARR’S INDEX
– FTIR
* EVALUATION OF TABLETS:-
– HARDNESS
– THICKNESS
– FRIABILITY
– WEIGHT VARIANCE
– IN VITRO DRUG RELEASE STUDIES
DRUG PROFILE
Candesartan:
Candesartan (rINN) (pronounced /kænd?s?rt?n/) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension. The prodrug candesartan cilexetil is marketed by AstraZeneca and Takeda Pharmaceuticals, commonly under the trade names Blopress, Atacand, Amias, and Ratacand.
Description:
Candesartan is an angiotensin-receptor blocker (ARB) that may be used alone or with other agents to treat hypertension. It is administered orally as the prodrug, candesartan cilexetil, which is rapidly converted to its active metabolite, candesartan, during absorption in the gastrointestinal tract. Candesartan lowers blood pressure by antagonizing the renin-angiotensin-aldosterone system (RAAS); it competes with angiotensin II for binding to the type-1 angiotensin II receptor (AT1) subtype and prevents the blood pressure increasing effects of angiotensin II. Unlike angiotensin-converting enzyme (ACE) inhibitors, ARBs do not have the adverse effect of dry cough. Candesartan may be used to treat hypertension, isolated systolic hypertension, left ventricular hypertrophy and diabetic nephropathy. It may also be used as an alternative agent for the treatment of heart failure, systolic dysfunction, myocardial infarction and coronary artery disease.
Structure:

Synonyms: candesartan cilexetil
Chemical formula: C24H20N6O3
Categories:
- Antihypertensive Agents
- Angiotensin II Receptor Antagonists
- Angiotensin II Type 1 Receptor Blockers
Molecular weight: 440.45
Melting point: 150 – 1600c
IUPAC Name: 2-ethoxy-1-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1H- ……………….1,3- benzodiazole-7-carboxylic acid
Indication:
May be used as a first line agent to treat uncomplicated hypertension, isolated systolic hypertension and left ventricular hypertrophy. May be used as a first line agent to delay progression of diabetic nephropathy. Candesartan may be also used as a second line agent in the treatment of congestive heart failure, systolic dysfunction, myocardial infarction and coronary artery disease in those intolerant of ACE inhibitors.
Pharmacodynamics:
Candesartan cilexetil is an ARB prodrug that is rapidly converted to candesartan, its active metabolite, during absorption from the gastrointestinal tract. Candesartan confers blood pressure lowering effects by antagonizing the hypertensive effects of angiotensin II via the RAAS. RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from granular cells of the juxtaglomerular apparatus in the kidneys. Renin cleaves circulating angiotensinogen to angiotensin I, which is cleaved by angiotensin converting enzyme (ACE) to angiotensin II. Angiotensin II increases blood pressure by increasing total peripheral resistance, increasing sodium and water reabsorption in the kidneys via aldosterone secretion, and altering cardiovascular structure. Angiotensin II binds to two receptors: type-1 angiotensin II receptor (AT1) and type-2 angiotensin II receptor (AT2). AT1 is a G-protein coupled receptor (GPCR) that mediates the vasoconstrictive and aldosterone-secreting effects of angiotensin II. Studies performed in recent years suggest that AT2 antagonizes AT1-mediated effects and directly affects long-term blood pressure control by inducing vasorelaxation and increasing urinary sodium excretion. Angiotensin receptor blockers (ARBs) are non-peptide competitive inhibitors of AT1. ARBs block the ability of angiotensin II to stimulate pressor and cell proliferative effects. Unlike ACE inhibitors, ARBs do not affect bradykinin-induced vasodilation. The overall effect of ARBs is a decrease in blood pressure.
Pharmacokinetics
Candesartan inhibits the binding of angiotensin II to AT1 receptors in many tissues such as vascular smooth muscles and adrenal gland which leads to vasoconstriction blockade and aldosterone release.
Absorption: Absorbed from the GI tract (oral); peak plasma concentrations after 3-4 hr.
Distribution: Protein-binding: 99%.
Metabolism: The prodrug candesartan cilexetil undergoes rapid and complete ester hydrolysis in the intestinal wall to form the active drug, candesartan. Elimination of candesartan is primarily as unchanged drug in the urine and, by the biliary route, in the feces. Minor hepatic metabolism of candesartan (<20%)occurs by O-deethylation via cytochrome P450 2C9 to form an inactive metabolite. Candesartan undergoes N-glucuronidation in the tetrazole ring by uridine diphosphate glucuronosyltransferase 1A3 (UGT1A3). O-glucuronidation may also occur. 75% of candesartan is excreted as unchanged drug in urine and feces.
Excretion: Via urine and bile (as unchanged drug and small amounts of inactive metabolites); 9 hr (elimination half-life).
Mechanism of action:
Candesartan selectively blocks the binding of angiotensin II to AT1 in many tissues including vascular smooth muscle and the adrenal glands. This inhibits the AT1-mediated vasoconstrictive and aldosterone-secreting effects of angiotensin II and results in an overall decrease in blood pressure. Candesartan is greater than 10,000 times more selective for AT1 than AT2. Inhibition of aldosterone secretion may increase sodium and water excretion while decreasing potassium excretion.
Candesartan Indications / Candesartan Uses
In hypertension and heart failure
Candesartan Adverse Reactions / Candesartan Side Effects
Dizziness, headache, vertigo, depression, somnolence, fever, back pain, upper respiratory tract infections, 1st dose orthostatic hypotension, rash, diarrhoea, decreased haemoglobin, renal impairment, hepatitis.
Special Precautions
Volume or sodium depletion, preexisting renal insufficiency; aortic or mitral valve stenosis, hypertrophic obstructive cardiomyopathy, renal artery stenosis, primary hyperaldosteronism. Patients with a history of angioedema, urticaria. Monitor serum potassium levels especially in elderly and renally impaired patients. Hypotension may occur during major surgery and anaesthesia due to suppression of the renin-angiotensin system.
Other Drug Interactions
Prior treatment with diuretics increases risk of excessive hypotension; use a lower initial dose. Potassium, potassium-sparing diuretics, trimethoprim or potassium supplements may increase risk of hyperkalaemia. NSAIDs may reduce antihypertensive response and increase risk of hyperkalaemia or acute renal failure. Excretion of lithium may be reduced; monitor lithium concentration.
Dosage
Oral
Hypertension
Adult: Initially, 8 mg once daily adjusted according to response. Maintenance: 8 mg once daily. Max: 32 mg daily.
Renal impairment: For patients with renal impairment or on haemodialysis: Initially, 4 mg once daily.
Hepatic impairment: Initially, 2 mg once daily. Avoid in severe impairment.
Oral
Heart failure
Adult: Initially, 4 mg once daily, may double dose at intervals of not <2 wk. Max: 32 mg daily. Renal impairment: Reduce dose or discontinue treatment if renal function deteriorates.
Hepatic impairment: Initially, 2 mg once daily. Avoid in severe impairment.
List of Contraindications
Candesartan and Pregnancy
Category C: Either studies in animals have revealed adverse effects on the foetus (teratogenic or embryocidal or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the foetus.
In 2nd & 3rd trimesters:
Category D: There is positive evidence of human foetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).
Candesartan and Lactation
It is not known whether candesartan passes into breast milk. However, candesartan passes into the milk of lactating rats. Because this medicine may cause serious side effects, breast-feeding is generally not recommended while you are taking it.
Candesartan and Children
Studies on this medicine have been done only in adult patients, and there is no specific information comparing use of candesartan in children with use in other age groups.
Candesartan and Geriatic
This medicine has been tested in patients 65 years of age or older and has not been shown to cause different side effects or problems in older people than it does in younger adults. However, blood levels of candesartan are increased in the elderly. Older people may be more sensitive than younger adults to the effects of candesartan.
Candesartan and Other Contraindications
Hypersensitivity. Pregnancy (2nd and 3rd trimester) and lactation
Storage
Oral: Store at 15-30°C
Sodium Starch Glycolate
1. Nonproprietary Names
BP: Sodium Starch Glycolate
PhEur: Sodium Starch Glycolate
USP-NF: Sodium Starch Glycolate
2. Synonyms
Carboxymethyl starch, sodium salt; carboxymethylamylum natricum; Explosol; Explotab; Glycolys; Primojel; starch carboxymethyl ether, sodium salt; Tablo; Vivastar
3. Chemical Name and CAS Registry Number
Sodium carboxymethyl starch [9063-38-1]
4. Empirical Formula and Molecular Weight
The USP32–NF27 describes two types of sodium starch glycolate, Type A and Type B, and states that sodium starch glycolate is the sodium salt of a carboxymethyl ether of starch or of a crosslinked carboxymethyl ether of starch.
The PhEur 6.0 describes three types of material: Type A and Type B are described as the sodium salt of a crosslinked partly Ocarboxymethylated potato starch. Type C is described as the sodium salt of a partly O-carboxymethylated starch, crosslinked by physical dehydration. Types A, B, and C are differentiated by their pH, sodium, and sodium chloride content.
The PhEur and USP–NF monographs have been harmonized for Type A and Type B variants.
Sodium starch glycolate may be characterized by the degree of substitution and crosslinking. The molecular weight is typically 5 x
105–1×106.
5. Structural Formula

6 Functional Category
Tablet and capsule disintegrant.
7 Applications in Pharmaceutical Formulation or Technology
- Sodium starch glycolate is widely used in oral pharmaceuticals as a disintegrant in capsule and tablet formulations. It is commonly used in tablets prepared by either direct-compression or wet granulation processes. The usual concentration employed in a formulation is between 2% and 8%, with the optimum concentration about 4%, although in many cases 2% is sufficient. Disintegration occurs by rapid uptake of water followed by rapid and enormous swelling. Although the effectiveness of many disintegrants is affected by the presence of hydrophobic excipients such as lubricants, the disintegrant efficiency of sodium starch glycolate is unimpaired.
- Increasing the tablet compression pressure also appears to have no effect on disintegration time.
- Sodium starch glycolate has also been investigated for use as a suspending vehicle.
8 Description
Sodium starch glycolate is a white or almost white free-flowing very hygroscopic powder. The PhEur 6.0 states that when examined under a microscope it is seen to consist of: granules, irregularly shaped, ovoid or pear-shaped, 30–100 mm in size, or rounded, 10–35 mm in size; compound granules consisting of 2–4 components occur occasionally; the granules have an eccentric hilum and clearly visible concentric striations. Between crossed nicol prisms, the granules show a distinct black cross intersecting at the hilum; small crystals are visible at the surface of the granules. The granules show considerable swelling in contact with water.
9 Typical Properties
Density (bulk)
0.756 g/cm3 for Glycolys;
0.81 g/cm3 for Primojel;
0.67 g/cm3 for Tablo.
Density (tapped)
0.945 g/cm3 for Glycolys;
0.98 g/cm3 for Primojel;
0.83 g/cm3 for Tablo.
Density (true)
1.56 g/cm3 for Primojel;
1.49 g/cm3 for Tablo.
Melting point Does not melt, but chars at approximately 2008C.
Particle size distribution 100% of particles less than 106 mm in size. Average particle size (d50) is 38mm and 42 mm for Primojel by microscopy and sieving, respectively.
Solubility Practically insoluble in methylene chloride. It gives a translucent suspension in water.
Specific surface area
0.24m2/g for Glycolys;
0.185m2/g for Primojel;
0.335m2/g for Tablo.


Swelling capacity In water, sodium starch glycolate swells to up to 300 times its volume.
Viscosity (dynamic) 4200 mPa s (200 cP) for a 4% w/v aqueous dispersion; viscosity is 4.26 mPa s for a 2% w/v aqueous dispersion (depending on source and grade).
10 Stability and Storage Conditions
Tablets prepared with sodium starch glycolate have good storage properties.Sodium starch glycolate is stable although very hygroscopic, and should be stored in a well-closed container in order to protect it from wide variations of humidity and temperature, which may cause caking.
The physical properties of sodium starch glycolate remain unchanged for up to 3 years if it is stored at moderate temperatures and humidity.
11 Incompatibilities
Sodium starch glycolate is incompatible with ascorbic acid.
12 Method of Manufacture
Sodium starch glycolate is a substituted derivative of potato starch. Typically, commercial products are also crosslinked using either sodium trimetaphosphate (Types A and B) or dehydration (Type C).
Starch is carboxymethylated by reacting it with sodium chloroacetate in an alkaline, nonaqueous medium, typically denatured ethanol or methanol, followed by neutralization with citric acid, acetic acid, or some other acid. Vivastar P is manufactured in methanolic medium, and Explotab in ethanolic medium.
13 Safety
Sodium starch glycolate is widely used in oral pharmaceutical formulations and is generally regarded as a nontoxic and nonirritant material. However, oral ingestion of large quantities may be harmful.
14 Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Sodium starch glycolate may be irritant to the eyes; eye protection and gloves are recommended. A dust mask or respirator is recommended for processes that generate a large quantity of dust.
Croscarmellose Sodium
1 Nonproprietary Names
BP: Croscarmellose Sodium
JP: Croscarmellose Sodium
PhEur: Croscarmellose Sodium
USP-NF: Croscarmellose Sodium
2 Synonyms
Ac-Di-Sol; carmellosum natricum conexum; crosslinked carboxymethylcellulose sodium; Explocel; modified cellulose gum; Nymcel
ZSX; Pharmacel XL; Primellose; Solutab; Vivasol.
3 Chemical Name and CAS Registry Number
Cellulose, carboxymethyl ether, sodium salt, crosslinked [74811- 65-7]
4 Empirical Formula and Molecular Weight
Croscarmellose sodium is a crosslinked polymer of carboxymethylcellulose sodium.
5 Structural Formula

6 Functional Category
Tablet and capsule disintegrant.
7 Applications in Pharmaceutical Formulation or Technology
Croscarmellose sodium is used in oral pharmaceutical formulations as a disintegrant for capsules, tablets, and granules. In tablet formulations, croscarmellose sodium may be used in both direct-compression and wet-granulation processes. When used in wet granulations, the croscarmellose sodium should be added in both the wet and dry stages of the process (intra- and extragranularly) so that the wicking and swelling ability of the disintegrant is best utilized. Croscarmellose sodium at concentrations up to 5% w/w may be used as a tablet disintegrant, although normally 2% w/w is used in tablets prepared by direct compression and 3% w/w in tablets prepared by a wet-granulation process.
8 Description
Croscarmellose sodium occurs as an odorless, white or grayishwhite powder.
SEM 1: Excipient: croscarmellose sodium (Ac-Di-Sol); manufacturer: FMC
Biopolymer; magnification: 100x.

SEM 2: Excipient: croscarmellose sodium (Ac-Di-Sol); manufacturer: FMC
Biopolymer; magnification: 1000x.

9. Typical Properties
Acidity/alkalinity pH = 5.0–7.0 in aqueous dispersions.
Bonding index 0.0456
Brittle fracture index 0.1000
Density (bulk) 0.529 g/cm3 for Ac-Di-Sol
Density (tapped) 0.819 g/cm3 for Ac-Di-Sol
Density (true) 1.543 g/cm3 for Ac-Di-Sol
10. Particle size distribution
Ac-Di-Sol: not more than 2% retained on a #200 (73.7 mm) mesh and not more than 10% retained on a #325 (44.5 mm) mesh.
Solubility Insoluble in water, although croscarmellose sodium rapidly swells to 4–8 times its original volume on contact with water. Practically insoluble in acetone, ethanol and toluene.
Specific surface area 0.81–0.83m2/g
11 Stability and Storage Conditions
Croscarmellose sodium is a stable though hygroscopic material.
A model tablet formulation prepared by direct compression, with croscarmellose sodium as a disintegrant, showed no significant difference in drug dissolution after storage at 308C for 14 months.
Croscarmellose sodium should be stored in a well-closed container in a cool, dry place.
12 Incompatibilities
The efficacy of disintegrants, such as croscarmellose sodium, may be slightly reduced in tablet formulations prepared by either the wet-granulation or direct-compression process that contain hygroscopic excipients such as sorbitol. Croscarmellose sodium is not compatible with strong acids or with soluble salts of iron and some other metals such as aluminum, mercury, and zinc.
13 Method of Manufacture
Alkali cellulose is prepared by steeping cellulose, obtained from wood pulp or cotton fibers, in sodium hydroxide solution. The alkali cellulose is then reacted with sodium monochloroacetate to obtain carboxymethylcellulose sodium. After the substitution reaction is completed and all of the sodium hydroxide has been used, the excess sodium monochloroacetate slowly hydrolyzes to glycolic acid. The glycolic acid changes a few of the sodium carboxymethyl groups to the free acid and catalyzes the formation of crosslinks to produce croscarmellose sodium. The croscarmellose sodium is then extracted with aqueous alcohol and any remaining sodium chloride or sodium glycolate is removed. After purification, croscarmellose sodium of purity greater than 99.5% is obtained. The croscarmellose sodium may be milled to break the polymer fibers into shorter lengths and hence improve its flow properties.
14 Safety
Croscarmellose sodium is mainly used as a disintegrant in oral pharmaceutical formulations and is generally regarded as an essentially nontoxic and nonirritant material. However, oral consumption of large amounts of croscarmellose sodium may have a laxative effect, although the quantities used in solid dosage formulations are unlikely to cause such problems. In the UK, croscarmellose sodium is accepted for use in dietary supplements.
Crospovidone
1.Nonproprietary Names
BP: Crospovidone
PhEur: Crospovidone
USP-NF: Crospovidone
2.Synonyms
Crospovidonum; Crospopharm; crosslinked povidone; E1202;
Kollidon CL; Kollidon CL-M; Polyplasdone XL; Polyplasdone
XL-10; polyvinylpolypyrrolidone; PVPP; 1-vinyl-2-pyrrolidinone homopolymer.
3.Chemical Name and CAS Registry Number
1-Ethenyl-2-pyrrolidinone homopolymer [9003-39-8]
4 Empirical Formula and Molecular Weight
(C6H9NO)n >1 000 000
The USP32–NF27 describes crospovidone as a water-insoluble synthetic crosslinked homopolymer of N-vinyl-2-pyrrolidinone. An exact determination of the molecular weight has not been established because of the insolubility of the material.
5 Structural Formula

6 Functional Category
Tablet disintegrant.
7 Applications in Pharmaceutical Formulation or Technology
Crospovidone is a water-insoluble tablet disintegrant and dissolution agent used at 2–5% concentration in tablets prepared by directcompression or wet- and dry-granulation methods. It rapidly exhibits high capillary activity and pronounced hydration capacity, with little tendency to form gels. Studies suggest that the particle size of crospovidone strongly influences disintegration of analgesic tablets. Larger particles provide a faster disintegration than smaller particles. Crospovidone can also be used as a solubility enhancer. With the technique of co-evaporation, crospovidone can be used to enhance the solubility of poorly soluble drugs. The drug is adsorbed on to crospovidone in the presence of a suitable solvent and the solvent is then evaporated. This technique results in faster dissolution rate.
8 Description
Crospovidone is a white to creamy-white, finely divided, freeflowing, practically tasteless, odorless or nearly odorless, hygroscopic powder.
9. Stability and Storage Conditions
Since crospovidone is hygroscopic, it should be stored in an airtight container in a cool, dry place.
10. Incompatibilities
Crospovidone is compatible with most organic and inorganic pharmaceutical ingredients. When exposed to a high water level, crospovidone may form molecular adducts with some materials.
11. Method of Manufacture
Acetylene and formaldehyde are reacted in the presence of a highly active catalyst to form butynediol, which is hydrogenated to butanediol and then cyclodehydrogenated to form butyrolactone. Pyrrolidone is produced by reacting butyrolactone with ammonia. This is followed by a vinylation reaction in which pyrrolidone and acetylene are reacted under pressure. The monomer vinylpyrrolidone is then polymerized in solution, using a catalyst. Crospovidone is prepared by a ‘popcorn polymerization’ process.
12. Safety
Crospovidone is used in oral pharmaceutical formulations and is generally regarded as a nontoxic and nonirritant material. Shortterm animal toxicity studies have shown no adverse effects associated with crospovidone. However, owing to the lack of available data, an acceptable daily intake in humans has not been specified by the WHO.
LD50 (mouse, IP): 12 g/kg
13 Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection, gloves, and a dust mask are recommended.
14 Regulatory Status
Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM injections, oral capsules and tablets; topical, transdermal, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Magnesium stearate
1 Nonproprietary Names
BP: Magnesium Stearate
JP: Magnesium Stearate
PhEur: Magnesium Stearate
USP-NF: Magnesium Stearate
2 Synonyms
Dibasic magnesium stearate; magnesium distearate; magnesia stearas; magnesium octadecanoate; octadecanoic acid, magnesium salt; stearic acid, magnesium salt; Synpro 90.
3 Chemical Name and CAS Registry Number
Octadecanoic acid magnesium salt [557-04-0]
4 Empirical Formula and Molecular Weight
C36H70MgO4 591.24
The USP32–NF27 describes magnesium stearate as a compound of magnesium with a mixture of solid organic acids that consists chiefly of variable proportions of magnesium stearate and magnesium palmitate (C32H62MgO4). The PhEur 6.5 describes magnesium stearate as a mixture of solid organic acids consisting mainly of variable proportions of magnesium stearate and magnesium palmitate obtained from sources of vegetable or animal origin.
5 Structural Formula
[CH3(CH2)16COO]2Mg
6 Functional Category
Tablet and capsule lubricant.
7 Applications in Pharmaceutical Formulation or Technology
Magnesium stearate is widely used in cosmetics, foods, and pharmaceutical formulations. It is primarily used as a lubricant in capsule and tablet manufacture at concentrations between 0.25% and 5.0% w/w. It is also used in barrier creams.
8 Description
Magnesium stearate is a very fine, light white, precipitated or milled, impalpable powder of low bulk density, having a faint odor of stearic acid and a characteristic taste. The powder is greasy to the touch and readily adheres to the skin.
9. Typical Properties
Crystalline forms High-purity magnesium stearate has been isolated as a trihydrate, a dihydrate, and an anhydrate.
Density (bulk) 0.159 g/cm3
Density (tapped) 0.286 g/cm3
Density (true) 1.092 g/cm3
Flash point 2508C
Flowability Poorly flowing, cohesive powder.
Melting range
117–150oC (commercial samples)
126–1308C (high purity magnesium stearate).
Solubility Practically insoluble in ethanol, ethanol (95%), ether and water; slightly soluble in warm benzene and warm ethanol (95%).
Specific surface area 1.6–14.8m2/g
Spectra of Candesartan by UV

No. P/V Wavelength(nm) Abs
1 Peak 255.00` 0.376
2 peak 217.00 0.915
1 Valley 243.00 0.342
STANDARD CALIBRATION CURVE
No. ID Type Conc [mg/l] Abs
λ max 217.00 nm
1 Standard1 Standard 0.0000 0.001
2 Standard2 Standard 1.0000 0.033
3 Standard3 Standard 2.0000 0.052
4 Standard4 Standard 3.0000 0.071
5 Standard5 Standard 4.0000 0.092
6 Standard6 Standard 5.0000 0.105
7 Standard7 Standard 6.0000 0.118

Preparation of tablets:
Preparation of blank tablet:
The diluent and the superdisintegrant were mixed thoroughly for five minutes. Subsequently, the homogeneous powder was combined with magnesium stearate as lubricant for two minutes. The mixed powder was compressed on tablet punching machine. Direct compression method was used to prepare tablets.
Drug solution dropped tablet (DSDT):
Candesartan was dissolved in a solvent of absolute alcohol or dichloromethane to obtain a solution of concentration of 100 micrograms per microliter. A 80-μl portion of the prepared solution (8mg candesartan) was dropped on the surface of direct compression blank tablet by using 50 μl-microsyringe (Hamilton # 80500). All of treated tablets were dried at 500c in hot air oven for one hour.
COMPOSITON OF VARIOUS TABLET FORMULATIONS:

Evaluation of Blend
Firstly compression into tablets, the blend was evaluated for following pre compression properties which includes parameters.
1. Angle of repose
Angle of repose was determined by using funnel method. Powder was poured from a funnel that can be raised vertically until a maximum cone height, h, was obtained. Diameter of heap, D, was measured. The angle of repose, ?, was calculated by formula
tan ? = h / r
? = tan-1 (h / r)
Where, ? is the angle of repose, h is the height in cm and r is the radius.
2. Bulk Density
Apparent bulk density was determined by pouring pre- sieved drug excipient blend into a graduated cylinder and measuring the volume and weight “as it is”. It is expressed in g/ml and is given by
Db = M / V0
Where, M is the mass of powder and V0 is the Bulk volume of the powder.
3. Tapped density
It was determined by placing a graduated cylinder, containing a known mass of drugexcipient blend, on mechanical tapping apparatus. The tapped volume was measured by tapping the powder to constant volume. It is expressed in g/ml and is given by
Dt = M / Vt
Where, M is the mass of powder and Vt is the tapped volume of the powder.
4. Porosity
The porosity € of powder is defined as the ratio of void volume to the bulk volume of the packaging.
The porosity of the powder is given by
€= Vb – Vp/ Vp =1- Vp/Vb
Porosity is frequently expressed in percentage and is given as
%€ = (1 – Vp/ Vb) X 100
5. Powder flow properties
The flow properties were determined by
i) Carr’s Index (I)
It is expressed in percentage and is expressed by
I = Dt – Db / Dt
Where, Dt is the tapped density of the powder and Db is the bulk density of the powder.
ii) Hausner ratio
It is expressed in percentage and is expressed by
H= Dt / Db
Where, Dt is the tapped density of the powder and Db is the bulk density of the powder.
Evaluation of tablets
Thickness and diameter
Thickness and diameter of tablets were determined using Vernier caliper. Five tablets from each batch were used, and an average value was calculated.
Hardness
The crushing strength of the tablets was measured using a Monsanto hardness tester. Three tablets from each formulation batch were tested randomly and the average reading noted.
Friability
Ten tablets were weighed and placed in a Roche friabilator and the equipment was rotated at 25 rpm for 4 min. The tablets were taken out, dedusted and reweighed. The percentage friability of the tablets was measured as per the following formula:
Initial weight – Final weight
Percentage friability = ———————————— x 100
Initial weight
Weight Variation
Randomly, twenty tablets were selected after compression and the mean weight was determined.
None of the tablets deviated from the average weight by more than ±4.5% (USP XX).
Drug content
10 tablets were weighed and powdered. An amount of the powder equivalent to 80mg of candesartan cilexetil was dissolved in 100ml of pH 7.4 phosphate buffer, filtered, diluted suitably and analyzed for drug content at 217nm using UV-Visible spectrophotometer.
Disintegration time
Disintegration time for DSDTs was determined using USP disintegration apparatus with phosphate buffer pH 7.4, 900 ml as the disintegrating medium at 37°C. To comply the test all tablets should disintegrate within 3 minutes.
In vitro dissolution studies
In vitro drug release studies of all the formulations were carried out using tablet dissolution test apparatus, (Labindia Electrolab, Mumbai) at 500rpm. Phosphate buffer pH 7.4 was used as the dissolution media with Samples were withdrawn at different intervals, diluted suitably and analyzed at 217–nm for cumulative temperature maintained at 37±1ºC. drug release using Shimadzu UV-Visible spectrophotometer.
Fourier transform infrared (FTIR) spectroscopy
Compatibility studies were carried out to know the possible interactions between Candesartan and excipients used in the formulation. Physical mixtures of drug and excipients were prepared to study the compatibility. Drug polymer compatibility studies were carried out using FTIR spectroscopy.
Scanning electron microscopy (SEM)
SEM photography was carried out to study the morphological character of the DSDT formulations, the surface, either superficial area or deeper area of DSDT was taken and analysed.
X-ray diffraction (XRD)
X-ray diffraction method was used to characterize the scratched powder from the tablet surface especially region of dropping drug solution of the DSDT.
Evaluation of blend
| Formulations | Bulkdensity gm/cm3 | Tapped Density gm/cm3 | Porosity (%) | Carrs index (%) | Angel ofRepose (0) | HausnerIndex (%) |
| F 01 | 0.33 | 0.50 | 66 | 34 | 19.42 | 1.5 |
| F 02 | 0.32 | 0.50 | 66 | 44 | 18.61 | 1.7 |
| F 03 | 0.29 | 0.47 | 65 | 35.4 | 19.20 | 1.5 |
| F 04 | 0.31 | 0.51 | 65 | 33 | 17.08 | 1.5 |
| F 05 | 0.33 | 0.49 | 66 | 34 | 18.21 | 1.7 |
| F 06 | 0.28 | 0.50 | 65 | 35.2 | 18.56 | 1.6 |
| F 07 | 0.34 | 0.51 | 66 | 38 | 19.60 | 1.7 |
| F 08 | 0.29 | 0.51 | 65 | 34 | 18.53 | 1.5 |
| F 09 | 0.31 | 0.47 | 65 | 34 | 17.09 | 1.5 |
| F 10 | 0.34 | 0.50 | 65 | 34 | 18.17 | 1.7 |
| F 11 | 0.30 | 0.49 | 65 | 35 | 17.41 | 1.6 |
| F 12 | 0.33 | 0.49 | 66 | 42.5 | 16.08 | 1.6 |
| F 13 | 0.33 | 0.51 | 66 | 33 | 17.58 | 1.6 |
| F 14 | 0.28 | 0.48 | 65 | 36 | 18.64 | 1.7 |
| F 15 | 0.31 | 0.48 | 66 | 33.5 | 17.56 | 1.5 |
| F 16 | 0.34 | 0.47 | 66 | 35 | 18.11 | 1.5 |
| F 17 | 0.28 | 0.51 | 65 | 33 | 18.63 | 1.7 |
| F 18 | 0.33 | 0.50 | 65 | 34.5 | 17.45 | 1.7 |
Evaluation of drug solution dropped tablets.
| Formulations | Hardness* (mm)±SD | Thickness* (mm)±SD | Friability* (%) | Weight variation* (mg)±SD | Disintegration time (sec) | % Drug release |
| F 01 | 2.25±0.25 | 2.08±0.11 | 0.28 | ±1.614 | 65 | 94.01 |
| F 02 | 2.42±0.16 | 2.17±0.15 | 0.35 | ±2.025 | 52 | 95.12 |
| F 03 | 2.56±0.53 | 2.06±0.08 | 0.34 | ±2.552 | 56 | 93.88 |
| F 04 | 2.35±0.24 | 2.25±0.16 | 0.45 | ±1.625 | 55 | 95.72 |
| F 05 | 2.65±0.37 | 2.35±0.20 | 0.33 | ±2.152 | 45 | 98.26 |
| F 06 | 2.45±0.34 | 2.19±0.19 | 0.29 | ±1.958 | 49 | 97.54 |
| F 07 | 2.22±0.38 | 2.10±0.14 | 0.36 | ±2.085 | 58 | 95.78 |
| F 08 | 2.63±0.26 | 2.26±0.10 | 0.46 | ±2.065 | 62 | 96.95 |
| F 09 | 2.35±0.45 | 2.20±0.15 | 0.39 | ±1.965 | 56 | 95.78 |
| F 10 | 2.39±0.29 | 2.23±0.19 | 0.42 | ±1.754 | 51 | 95.79 |
| F 11 | 2.50±0.31 | 2.19±0.21 | 0.55 | ±2.645 | 64 | 96.36 |
| F 12 | 2.58±0.37 | 2.15±0.12 | 0.44 | ±1.851 | 66 | 94.59 |
| F 13 | 2.44±0.25 | 2.29±0.15 | 0.42 | ±2.115 | 55 | 96.36 |
| F 14 | 2.36±0.45 | 2.35±0.19 | 0.36 | ±1.865 | 52 | 97.55 |
| F 15 | 2.41±0.28 | 2.32±0.08 | 0.27 | ±1.995 | 61 | 96.37 |
| F 16 | 2.56±0.36 | 2.06±0.11 | 0.33 | ±2.105 | 59 | 96.96 |
| F 17 | 2.29±0.44 | 2.15±0.14 | 0.48 | ±2.187 | 55 | 95.76 |
| F 18 | 2.48±0.26 | 2.09±0.21 | 0.37 | ±1.989 | 52 | 95.75 |
DISSOLUTION STUDIES OF VARIOUS FORMULATIONS


FTIR Analysis:

Figure : FTIR Spectrum of candesartan pure drug

Figure : FTIR Spectrum of candesartan + sodium starch glycolate

Figure : FTIR Spectrum of candesartan + crosscarmellose sodium

Figure : FTIR Spectrum of candesartan + cross povidone

Figure : FTIR Spectrum of candesartan + CCS + MCC + Mg. Stearate

Figure : FTIR Spectrum of candesartan + SSG + DCP + Mg. Stearate
DSC thermograms

DSC thermogram of Candesartan + SSG + MCC + Mg.Stearate
Morphology studies
Optimized formula


XRD Analysis


DISCUSSION
DSDT purpose the advantage over conventional tablet.
The advantages are as followings:
1) There are no requirements on the compressibility of the active ingredients
2) The exact concentration of drug solution dropping on the blank tablet, resulting in a more uniform dispersion of fine particles; and
3) The bioavailability of the drug substance could be improved when it is dispersed at the molecular level in drug solution.
By SEM method it could not point out candesartan particles from the surface, either superficial area or deeper area of DSDT from the other excipients of blank tablet. It might be of very small size occurred after solvent evaporation and of extremely small amount of drug.
FTIR and DSC analysis results revealed that there are no interactions between active ingredient and specific excipients.
For XRD method, the intensity of X-ray diffraction peak of the powder scratched from the surface of candesartan-solution-dropped tablets prepared from DC. It was clearer when using single-crystal X-ray diffraction method to characterize the scratched powder from the tablet surface especially region of dropping drug solution.
CONCLUSION
In conclusion it was suggested that DSDT could be a new suitable process for the preparation of tablet dosage form for poorly water soluble drug or other therapeutic substances especially for the low dose drugs. It was appropriate method for some drug which their physical properties such as crystallinity was altered or changed under mixing; compression force or uniformity including its poor compressibility was exactly needed. To solve this problem this preparation was started by using DC blank tablet and then introduced an active ingredient in liquid form into the blank tablet.
The DSDT5 formula (with 6% SSG, 3% Mg.Stearate, MCC as filler and Dichloromethane as solvent) has shown the better drug release i.e 98% during invitro evaluation studies and also shown good post compression parameters so it was optimised.
BIBLIOGRAPHY
1.Orawan Chitvanich, 2009 Preparation and characterization of drug solution dropping tablet, CMU. J . Nat. Sci.(2009) Vol. 8(2) 175.
2.Orawan Chitvanich, 2008 Preparation and characterization of chlorpheniramine maleate-solution-dropping tablet, CMU. J . Nat. Sci.(2008) Vol. 7(1) 73.
3.James K; “Solubility and related properties”, vol. 28, Marcel Dekker, Newyork, 986, pp.127 –146, 355 – 395.
4.Indian Pharmacopoeia, Ministry of Health and family welfare, Government of India, Published by the controller of publications, Delhi, 1996, 1, 7.
5.Pinnamaneni S, Das N G, Das S K; Formulation approaches for orally administered poorly soluble drugs. Pharmazie, 2002; 57: 291 – 300.
6.Chaumeil J C; Micronisation: A method of improving the bioavailability of poorly soluble drugs, Methods and Findings in Experimental and Clinical Pharmacology, 1998; 20:211-215.
7.Aulton M E; Pharmaceutics, The science of dosage form design, 2nd edition, Churchill Livingstone, London, 2002; pp.113 – 138, 234 – 252.
8.Banga S, Chawla G, Bansal A K; New Trends in the Crystallisation of Active Pharmaceutical Ingredients, Businessbriefing: Pharmagenerics, 2004; 70-74.
9.Rawat A, Jaitely V, Kanaujia P et.al. Supercritical Fluid Technology perspectives and industrial application. The Eastern Pharmacist. 1994; 12: 35-43.
10.Pasquali I, Bettini R, Giordano F; Solid-state chemistry and particle engineering with supercritical fluids in pharmaceutics, European journal of pharmaceutical sciences 2006; 27:299–310.
11.Sekiguchi K, Obi N; Studies on absorption of eutectic mixtures. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man, Chem. Pharm. Bull., 1961; 9: 866-872.
12.Rajewski R A, Stella V J; Pharmaceutical applications of cyclodextrins, In vivo drug delivery, J. Pharm. Sci., 1996; 85:1142-1169.
13.Park J Woo; Kinetics and Mechanism of Cyclodextrin Inclusion Complexation Incorporating Bidirectional Inclusion and Formation of Orientational Isomers, J. Phys. Chem. B, ASAP Article, 2005; 32:124-127.
14.Mosher G, Thompson D O; Complexation and cyclodextrins, in “Encyclopedia of pharmaceutical technology”, 2nd edition, Marcel Dekker Inc., Newyork, 2002; 1,531 – 558.
15.Swarbrick J, Boylan J C; Encyclopedia of Pharmaceutical Technology; 2nd edn, 2002, 2458-2479.
16.Tenjarla S N; Microemulsions: An overview and pharmaceutical applications. Critical Reviews in Therapeutic Drug Carrier Systems, 1999; 16: 461-521.
17.Agharkar S, Lindenbaum S, Higuchi T; Enhancement of solubility of drug salts by hydrophilic counter-ions: properties of organic salts of an anti-malarial drug, J. Pharm.Sci., 1976; 65: 747-749.
18.Almarsson O, Zaworotko M J; Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem. Commun., 2004; 1889-1896.
19.Lachman L, Liberman H A, Kanig J L; The Theory and Practice of Industrial Pharmacy, Verghese Publishing, Indian edition, 3rd edn.,1987;462-466.
20.Babu M M, Prasad D S, Ramana Murthy K V; Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine, International Journal of Pharmaceutics, 2002; 234: 1–17.
21.Valizadeh H, Nokhodchi A, Qarakhani N, etal.Physicoch-emical characterization of solid dispersions of indomethacin PEG 6000, Myrj 52, lactose, sorbitol, dextrin and Eudragit E100. Drug Dev Ind Pharm. 2004; 30: 303-317.
22.Keck C M, Muller R H; Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm., 2006; 62: 3-16.
23.Kondo N, Iwao T, Masuda H et al. Improved oral absorption of a poorly water-soluble drug, HO-221, by wet-bead milling producing particles in submicron region. Chem Pharm Bull, 1993; 41: 737-740.
24.Gershanik T, Benita S; Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs, Eur. J. Pharm. Sci. Biopharm. 2000; 50: 179–188.
25.Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K; Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm, 2005; 299:167-177.
26.Chung H R, Kwon E, Oikawa H, Kasai H et al , Effect of solvent on organic nanocrystal growth using the reprecipitation method. J Cryst Growth, 2006; 2: 459-463.
27.Salvadori B, Capitani G C, Mellini M et al , A novel method to prepare inorganic water-soluble nanocrystals. J Colloid Interface Sci, 2006; 1:487-490.