Introduction
Early work on transdermal drug delivery (TDD) goes back to the 1960s, and in 1971 the seminal US Patent titled “Bandage for Administering Drugs” was granted to ALZA Corporation.1 Since then, significant investments into research and development of TDD have resulted in a strong increase in scientific output, with more than 1000 scientific articles and book chapters on this topic in the calendar year 2013 alone. Today, transdermal medicines, like the fentanyl patch and others, provide significant patient benefits. Correspondingly, the global market for TDD is attractive and growing. It generated revenues of approximately $25 bn in 2013 and is expected to exceed $40 bn by 2018.2
Yet, despite 5 decades of innovation in the area of TDD and its high acceptance by patients, only 23 molecular entities (MEs) out of approximately 2500 MEs approved for human use in the US3 are available in the form of TDD systems.4
Given these 3 factors (5 decades of TDD research and development, general market acceptance of transdermal systems [TSs], and, in particular, patient benefits from TDD), one question comes to mind:
How can we provide more of these valuable medicines to patients in need?
In order to address this question, this article will briefly discuss the status quo of TDD, provide an overview of challenges in the creation of new transdermal medicines, and then focus on opportunities for the future.
Status Quo of TDD
Five decades of research and development in the area of TDD have been fundamentally driven by several basic advantages of the transdermal route for the administration of certain MEs. These advantages include effective control of blood levels, low frequency of application, circumvention of the first liver passage, avoidance of gastrointestinal inactivation, ease of treatment termination, and noninvasive administration.
Effective Control of Blood Levels
An effective control of blood levels is especially important for drugs with a narrow therapeutic index, such as nitroglycerin and fentanyl.5 After application of a TS and a short lag-time6 an increase in drug concentration occurs in the plasma. Ideally, plasma drug levels rapidly exceed the lower threshold of the therapeutic window and then continue to increase until a plateau is reached where drug levels are sustained for a prolonged time. Figure 1 shows a schematic concentration-time curve upon application of a TS. Figure 1. Systemic concentration of a ME after administration of a TS.
Figure 1. Systemic concentration of a ME after administration of a TS.
Low Frequency of Application
Transdermal administration, even of MEs with short biologic half-life, can be effectively achieved: once-a-day (like Transderm-Nitro®),7 twice-a-week (like Estraderm®),8 or once-a-week (like ClimaraPro®)9 systems have been developed to date. ClimaraPro® simultaneously delivers the steroids levonorgestrel and estradiol, the latter of which has a short biological half-life of approximately 0.5 h, at therapeutically relevant plasma levels over the course of 7 days. The low frequency of application of once-a-week systems helps in achieving improved patient compliance in chronic therapies.
Circumvention of the First Liver Passage
The liver is the main organ of metabolism for most MEs and many drugs are subject to a high first-pass metabolism, once applied orally. One example is estradiol, which is almost completely absorbed after oral administration but extensively metabolized by the first liver passage. Only about 5% of the administered dose becomes systemically available. Transdermal application of the steroid helps to significantly reduce the daily delivered dose and thus the liver burden.
Protection of Labile Drugs Against Gastrointestinal (GI) Inactivation
Acid-labile drugs are often rapidly decomposed during the passage of the GI tract due to the low pH values in the stomach and thus are no longer available in their entirety for absorption. Transdermal application avoids that and can thereby increase bioavailability of the drug.
Secure and Easy Termination of Treatment
The patient can interrupt the therapy by removing the TS at any time. After removal of the system, the active substance concentration in the blood falls as a function of the amount of drug remaining in the skin depot and of the biological half-life of the drug itself.
Non-invasive Drug Administration
Transdermal application (as opposed to a continuous infusion) makes hospitalization of patients unnecessary. TDD provides specific benefits in areas like quality of life,10 acceptance, compliance, safety, and patient treatment costs.11
From a technological perspective, the early focus of transdermal research and development lay on passive transdermal delivery of MEs as with reservoir and matrix type systems complemented by gel/liquid formulations. These systems often contain penetration enhancers in order to allow for sufficiently high transdermal fluxes of the MEs. Later on, active systems have created significant interest like the recently FDA-approved sumatriptan iontophoretic transdermal system for patients suffering from migraines.
Figure 2 provides a simultaneous overview of scientific output (number of journal articles and book chapters globally–patents and abstracts not included) and of marketed TSs output. It is important to note that the latter only include the first FDA-approved TS for a specific ME, like Estraderm® (approved by the FDA in 1986) for the ME estradiol. It neither contains subsequent introductions of transdermal dosage forms with the same ME nor does it contain any liquid or gel-based transdermal products. For an in-depth overview of the transdermal product landscape, please refer to the excellent reviews by Prausnitz and Langer7 or Paudel et al.8 Zoom In
Zoom In
Figure 2. Five decades of TDD innovation.
Figure 2 shows that both the scientific as well as the product output in the transdermal area have doubled in the recent decade. This in part explains the aforementioned significant growth of the TDD market, which reached the size of approximately $25 bn in 2013 and is expected to exceed $40 bn by 2018. However, market growth is not driven only by the introduction of novel systems based on additional MEs, but also by the addition of subsequent products based on MEs with already available transdermal products. This provides patient benefits and thus triggers additional market growth. Table 1 shows FDA-approved transdermal medications based on estradiol alone where Estraderm® was the first product to obtain approval and 10 additional products followed.
Table 1. FDA-approved transdermal products for prolonged systemic estradiol delivery

As Table 1 shows, continued investment into the development of transdermal dosage forms for a specific ME over an extended period of time results in new products that provide the individual patient with a choice. In the case of estradiol, products now span from matrix-over-reservoir-systems, to gels, to a spray, and provide application intervals from daily, to twice-a-week, and to once-a-week. Comparable transdermal product families based on the same ME exist today (eg, nicotine and nitroglycerin).
Passive TSs, like the matrix and reservoir-type TSs, gels and liquid formulations mentioned above, helped with the delivery of certain highly active MEs with low molecular weight and moderate lipophilicity. However, these systems are typically not capable of delivering peptides, proteins, or even small molecule drugs which carry charges at physiologic pH values.
Given the vast therapeutic potential of MEs stemming from these compound classes, in particular proteins and peptides, significant efforts have been made to research and develop the area of active and minimally invasive transdermal delivery over the past 3 decades. Table 2 provides examples of technologies and MEs in that regard.
Table 2. Examples of active and minimally invasive transdermal delivery technologies

Most of the work on non- and minimally invasive transdermal drug delivery is still in preclinical and clinical development stages and only a few products achieved FDA approval to date.
Challenges of TDD
Limitations of TDD arise primarily from the structure and function of the skin, in particular its barrier function. The skin of an adult is about 3 mm thick. It protects the body against chemical, microbial, and physical effects of the environment. Additionally it contributes significantly to the regulation of body temperature and water balance. The barrier function, which is of utmost importance for the health of patients, is also responsible for the fact that only comparatively low percutaneous absorption rates can be achieved upon transdermal application of most MEs.
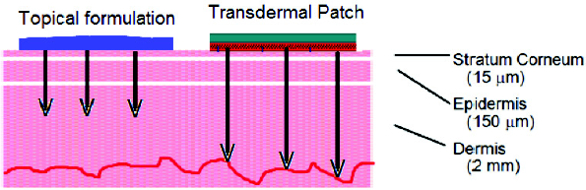
The outermost layer of the skin, the stratum corneum is approximately 20 μm thick and consists of a brick and mortar like structure of corneocytes surrounded by intercellular lamellar lipids (see Figure 3). It is typically permeable by moderately lipophilic compounds only. Figure 3. Schematic view of the skin.
Figure 3. Schematic view of the skin.
Underneath the stratum corneum lies the viable epidermis, which is hydrophilic in nature. Any compound which has crossed the stratum corneum needs to be sufficiently hydrophilic to permeate the viable epidermis and to be taken up into the systemic blood circulation subsequently.
The barrier function of the skin reduces the suitability of the transdermal route to an extremely limited set of therapeutic moieties. As mentioned above, only 23 MEs are approved for transdermal administration in the US out of a pool of over 2500 FDA-approved MEs, presenting an odds ratio of below 1 in 1000 for identifying a suitable transdermal drug candidate and designing the corresponding delivery system. The currently approved systems deliver their respective MEs via sophisticated systems and formulations, mostly by transiently reducing the barrier function of the skin for the penetrant to be absorbed. However, manipulating the skin barrier during transdermal application of drugs can increase the risk for local adverse reactions. Skin irritation—and in certain cases—allergic reactions may occur. The potential for local adverse reactions is a significant challenge of TDD.
Further challenges of TDD are driven by the complexity of the technological approaches selected to solve a specific delivery problem, like the construction of a device for electroporation, sonophoresis, or iontophoresis and the complementary development of the accompanying non-irritating and stable formulation for the delivery of a certain ME.
In addition, patient usability of the TS presents other challenges. Matrix- and reservoir-patches, for example, need to be developed towards low rates of detachment and patch loss, which is particularly challenging in the area of once-a-week systems. Transdermal gels, on the other hand, need to be developed so that the potential transfer of the active ingredient to third persons is limited. Ease of use by the patient and minimization of skin irritation is of utmost importance in the development of complex devices. These are only a few examples of the complexities a drug development team might face during the course of creating a novel transdermal medicine.
Opportunities of TDD
In analyzing the progress made over the past 5 decades, it becomes clear that 2 main levers have been utilized for the creation of opportunities for new transdermal medications:
- Identifying suitable MEs from the pool of approved MEs
- Reducing the barrier function of the skin–particularly of the stratum corneum—by chemical (penetration enhancer), biological (occlusion/hydration), or physical (ultrasound, heat, etc.) means

A closer look at the examples of transdermal medicines in use today highlights the fact that drug development teams will typically combine the use of the aforementioned levers as they create their products. Going forward, it remains to be seen to what extent the investments into various forms of active, minimally, and non-invasive administration of therapeutic moieties will enable novel transdermal medicines. The demand from the patient side for non- or minimally invasive delivery of certain therapeutic macromolecules is substantial, and today maturing technologies like microneedles look very promising in that regard. It is likely that future introductions of novel transdermal medicines enabled by such innovative technologies will trigger a new wave of approved transdermal products. Therefore, building on the past and investing in the levers of technology as well as identification of suitable MEs will continue to provide opportunities for valuable new products. Yet, at a strategic level, 2 important levers seem to be underexploited as of today:
- Design of transdermal prodrugs of approved MEs
- De novo design of MEs based on transdermal developability considerations
Historically, life cycle management of already introduced medicines and the utilization of the 505(b)(2) regulatory pathway has been the main route used for the introduction of novel transdermal product. This is a very sensible approach, given that it minimizes risk for failure and technical attrition due to potential problems with an unproven ME. Yet this approach has focused on a set of compounds which were more suitable for oral or parenteral administration in most cases—application routes which are significantly different from the transdermal route. From a theoretic vantage point, applying developability criteria in drug design—comparable to the rule-of-five12 in the oral drug design domain—based on the specific requirements for TDD, should increase the number of suitable compounds. This could be done both via a prodrug approach and a de novo approach, with the latter providing more flexibility but also significantly more risk for attrition. While there is a remarkable body of scientific work on transdermal delivery of prodrugs,13,14 mainly driven by researchers in academia, little has been reported about the latter approach of specifically designing new therapeutic moieties for the transdermal route of administration. This is in part understandable, due to the patient preference for oral administration of drugs in many therapeutic areas. Yet it should offer some opportunity for drug finding programs, where the chemical scaffold does not lend itself to oral bioavailability and non-invasive application with longer dosing intervals is required. While 20 years ago, the aggregate risk for technological attrition might have seemed too high to devote a de novo drug design program to the transdermal route, recent advances in the transdermal delivery sciences have reduced the delivery related risk for attrition today. Taking the latter approaches—designing prodrugs and de novo medical entities for transdermal administration—also poses a significant challenge from an organizational standpoint, given the historical division of labor with pharmaceutical companies mostly focusing on de novo drug design and drug delivery companies specializing in sophisticated delivery technologies. The fundamental touch point between these ventures often has been the collaboration on line extensions for existing MEs. While the historical division of labor in the broad area of TDD has been effective to a certain degree a fundamentally new paradigm, bridging transdermal prodrug and drug design and delivery, has the potential for providing more useful medicines in the future. New types of business models or ventures might be required to enable of innovations of this type.
Summary
Significant technological progress has been made in the area of passive, active, and minimally invasive transdermal delivery technologies. This very progress has resulted in products providing great patient benefits and simultaneously a significant market has been created. Yet remarkably, these benefits have been derived from a very limited set of therapeutically active moieties. Less than 1 in 1000 approved therapeutic moieties has been developed into marketed transdermal products to date.
The central question raised at the outset of this article ‘How can we provide more of these valuable medicines to patients in need?’ may be answered as follows:
- In the short-term, continued use of the lever of selection of MEs from the pool of approved MEs and developing corresponding tailor-made delivery systems can make additional products available.
- In the mid-term, technological progress in the area of delivery—particularly active and minimally invasive technologies—has the potential to generate a second wave of transdermal products, including those delivering macromolecules.
- In the long-term, bridging prodrug and drug design with drug delivery science to create a larger pool of suitable entities for transdermal delivery holds remarkable promise.
The latter approach asks for significant sophistication and inherently carries a higher risk of attrition, yet the opportunity space it opens is substantial. New types of business models or ventures might be required to realize these kinds of opportunities.
All in all, while 5 decades of innovation led to 23 MEs approved for use in TSs, evidence suggests it will not take 5 additional decades to develop the next set of 23 MEs into FDA-approved transdermal medicines.
Author Biography
Ralph Lipp, PhD is President and CEO of Lipp Life Sciences LLC. Before founding Lipp Life Sciences LLC, he served as Vice President Pharmaceutical Sciences R&D at Eli Lilly and Company and Head Pharmaceutical Development at Schering AG. Ralph holds a degree in Pharmacy from Johannes Gutenberg University in Mainz and obtained a PhD in Medicinal Chemistry as well as a Habilitation for Pharmaceutical Technology from Free University Berlin. He graduated from INSEAD’s International Executive Program as well as from Harvard Business School’s Advanced Management Program. Ralph’s scientific contributions comprise over 120 publications including more than 20 patents, covering five marketed medicines including one transdermal patch.
References
- Zaffaroni A, inventor; Bandage for administering drugs. U.S. Patent 3,598,122. August 10, 1971.
- Transdermal Drug Delivery Market & Clinical Pipeline Insight. Research and Markets website.http://www.researchandmarkets.com/reports/2823029/transdermal-drug-deliverymarket- and-clinical. Accessed June 24, 2014.
- Huang R, Southall N, Wang Y, et al. The NCGC Pharmaceutical Collection: A comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med. 2011; 3(80):16.
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration website. http://www.accessdata.fda.gov/scripts/cder/ob/. Updated May 2014. Accessed June 24, 2014.
- Dollery C, ed. Therapeutic Drugs. Vol. 1, 2 & suppl., Edinburgh, London, Melbourne: Churchill Livingstone; 1991.
- Barry BW. Dermatological formulations. In: J. Swarbrick J, ed. Drugs in the pharmaceutical sciences. Vol. 18. New York, NY, USA and Basel, Switzerland: Marcel Dekker Inc.; 1983: 188-189.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261-1268.
- Paudel KS, Milewski M, Swadley CL, Brogden NK, Gosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109-131.
- Harrison LI, Zurth C, Günther C, Karara AH, Melikian A, Lipp R. Simultaneous estradiol and levonorgestrel transdermal delivery from a 7-day patch: in vitro and in vivo drug deliveries of three formulations. Drug Dev Ind Pharm. 2007;33:373-380.
- Payne R. Factors influencing quality of life in cancer patients: the role of transdermal fentanyl in the management of pain. Seminars in Oncology. 1998;25(suppl 7S):47-53.
- Bloor K, Leese B, Maynard A. The costs of managing severe cancer pain and potential savings from transdermal administration. Eur J Cancer. 1994;30(Part A: General Topics):463-468.
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies.2004;1(4):337-341.
- Sloan KB, Devarajan-Ketha H, Wasdo SC. Dermal and transdermal delivery: prodrugs. Therap Deliv. 2011;2(1):83-105.
- Lipp R, Laurent H, Günther C, Riedl J, Esperling P, Täuber U. Rational design of prodrugs for matrix-type transdermal delivery systems: gestodene esters. Pharm Res. 1998;15:1419-1424.