Abstract
Background: Our goal was to prepare and evaluate topical ophthalmic formulations containing optimized celecoxib-loaded bioadhesive cationic chitosan or anionic alginate nanoparticles for sustained release of celecoxib.
Methods: Nanoparticles were prepared using a spontaneous emulsification solvent diffusion method. Different concentrations of polymers, emulsifier and stabilizers were used to optimize our formulations. The formulation that gave the lowest particle size and polydispersity index and the highest zeta potential was selected and subjected to further studies. The optimized celecoxibloaded NPs were characterized with regard to their particle size, polydispersity index, zeta potential, morphology and drug content. Celecoxib-loaded NPs were incorporated in topical ophthalmic dosage forms including eye drops, temperature-triggered in situ gelling system and preformed gel. All formulations were then characterized regarding their pH, viscosity, uniformity of drug content, in vitrorelease study and in vitro cytotoxicity.
Results: Results of the optimization studies of both celecoxib-loaded chitosan and alginate nanoparticles respectively, are: particle size of 113.33 ± 4.08 nm and 154.67 ± 5.06 nm; zeta potential of +36.92 ± 3.38 mV and -36.5 ± 4.7 mV; and encapsulation efficiencies of 89.88 ± 4.17% and 75.38 ± 2.98%. Transmission electron microscopic analysis revealed that all nanoparticles have distinct spherical shapes comprising of a solid dense core covered with evenly distributed coat. oreover, all formulations possessed pH and viscosity values that are compatible with the eye and have uniform drug contents that complied with the US Pharmacopeial (USP) official requirement. In vitro release data of ophthalmic formulations showed a sustained release without any burst effect and the formulations followed a Higuchi non-Fickian diffusion mechanism. The results of in vitrocell toxicity revealed that all the prepared formulations are non-toxic, as the percentage cell viability ranged from 89.9 to 97.7%.
Conclusions: These formulations provide a great deal of flexibility to the formulation scientist whereby the sizes and zeta potentials of the formulations can be tuned to suit the need using scalable and robust methodologies. This could thus serve as a potential drug delivery system for both the anterior and posterior eye diseases.
Keywords: Chitosan, alginate, ophthalmic, celecoxib, nanoparticles, formulations, cytotoxicity
Background
One of the most attractive aspects of drug delivery research is the ability to design nanocarriers that are able to deliver drugs to the right place, at the appropriate times and at accurate concentrations.These nanocarriers are nanoparticulate systems that entrap drugs and may prevent or minimize drug degradation and metabolism, which possibly increases cellular uptake [1]. Nanoparticulates have many advantages such as a long shelf life, being made from safe materials–including synthetic and natural biodegradable polymers, lipids and polysaccharides–and having the ability to pass important mucosal barriers, such as the intestinal, nasal and ocular barriers [2].
A major drawback of conventional topical ophthalmic drug delivery systems is the rapid and extensive loss of drug caused by the drainage through the nasolachrymal duct and high tear fluid turnover [3]. Several studies have attempted to increase the corneal penetration of drugs using colloidal drug delivery systems, such as liposomes [4], nanoparticles [5] and nanocapsules [6], which would in turn improve the therapeutic effect. Unfortunately, the short residence time of these colloidal carrier systems on the ocular surface remains a major challenge for the therapy of diseases of the external portions of the eye. Consequently, the design of a mucoadhesive nanocarrier system with improved drug delivery properties toward the ocular surface would be a promising step towards the treatment these diseases. It is predicted that the use of mucoadhesive polymers, which may interact with cornea and conjunctiva, will increase the concentration and residence time of its associated drug. Among the wide variety of natural mucoadhesive polymers reported in the literature, chitosan (CS) and sodium alginate (ALG) are the most widely used, safe, biocompatible and biodegradable ones [7].
Chitosan, [α(1-4)2-amino 2-deoxy β-D glucan] (CS), is a deacetylated form of chitin, a polysaccharide present in abundance in the shells of crustaceans [8]. It has been a polymer of choice because of its unique properties including bioadhesiveness, biodegradability, biocompatibility [9], as well as its penetration enhancing properties in both in vitro and in vivo conditions [10]. Its biodegradability is due to its degradation by lysozyme, an enzyme that is available in serum [11] and is highly concentrated in the lacrimal fluid [12]. Studies have shown that CS also exhibits antibacterial activity [13]. Finally, CS has recently been proposed as a material with a good potential for topical ocular drug delivery due to its ability to prolong the corneal residence time, as CS nanoparticles can remain attached to the cornea and theconjunctiva for 24 h [14].
Alginates (ALG) are random, linear and anionic polysaccharides consisting of linear monomers, α-L-guluronic acid and β-D-mannuronic. Alginates have a long history of use in numerous biomedical applications, including drug delivery systems, as they are biodegradable, biocompatible and mucoadhesive polymers [1]. Alginate polymers are also safe and hemocompatible. They do not accumulate in any major organs and show evidence of in vivodegradation [15]. Sodium alginate is used in a variety of oral, topical and ophthalmic pharmaceutical formulations and it has been specifically used for the aqueous microencapsulation of drugs instead of the conventional solvent based systems [16]. Due to its previously mentioned properties, ALG is considered as an excellent polymer for preparation of biodegradable, biocompatible and mucoadhesive nanoparticles for sustained ophthalmic drug delivery [17].
Non-steroidal anti-inflammatory drugs have been used topically to manage some ophthalmic problems such as enhance mydriasis, reduce postoperative inflammation, and prevent and treat cystoid macular edema associated with cataract surgery. In addition, they can be used to decrease pain and photophobia after refractive surgery and to alleviate itching associated with allergic conjunctivitis [18]. Celecoxib exerts its action through a selective inhibition of the cyclooxygenase-2 enzyme [19]. In addition to its anti-inflammatory role, celecoxib decreases the expression of vascular endothelial growth factor, which makes it a plausible therapy for proliferative diabetic retinopathy, neovascular age-related macular degeneration [20] and some ocular tumors such as retinoblastoma [21] and metastatic uveal melanoma [19].
The most common route of administration for celecoxib in treatment of ocular diseases is oral [22]. This route, however, has many drawbacks including that the amount of drug that reaches the site of action is very low, thus requiring a high dose for a long period of time, which may increase the risk of occurrence of side effects [23]. Several trials have attempted to overcome the drawbacks of its systemic use. For example, Amrite et al., prepared celecoxib in the form of periocular injectable microparticles for treatment of diabetes-induced elevation in retinal prostaglandins, vascular endothelial growth factor, and vascular leakage [24]. In another study, Ayalasomayajula and Kompella prepared subconjunctivally administered celecoxib-poly (D,L- lactide-co-glycolide) (PLGA) microparticles for sustained retinal drug delivery levels for treatment of diabetes-induced oxidative stress in a rat model [25]. Lastly, Cheruvu et al., prepared celecoxib in two injectable forms i.e., celecoxib suspension in 0.5% carboxymethylcellulose and celecoxib-loaded poly(L-lactide) (PLA) nanoparticles to study the effect of eye pigmentation on the trans-scleral drug delivery to the retina from both rapid release and sustained release formulations [26]. While these studies showed increased delivery of celecoxib to the posterior segment of the eye, they all required invasive delivery methods. In our study, we sought to formulate celecoxib topical ophthalmic formulations to be administered non-invasively for treatment of both anterior and posterior eye diseases.
In the present study, we report a modified spontaneous emulsification/solvent diffusion method to prepare cationic CS and anionic ALG celecoxib-loaded nanoparticles (NPs) for topical ophthalmic use. We then incorporated the drug-loaded nanoparticles in three different ophthalmic dosage forms including eye drops, a temperature triggered in situ gelling system and a preformed gel. The current study was aimed at developing and optimizing sustained release, mucoadhesive and biodegradable nanoparticles formulations of celecoxib for topical ocular delivery.
Methods
Materials
Celecoxib, hydroxypropylmethylcellulose (HPMC), methylcellulose (Methocel, MC), polyvinyl alcohol (PVA, molecular weight 31,000 – 50,000 Da), Triton X-100, methyl thiazol tetrazolium (MTT), sodium chloride, potassium chloride, sodium phosphate dibasic, potassium dihydrogen phosphate, absolute ethyl alcohol, acetone and dichloromethane (DCM) were purchased from Sigma-Aldrich (St. Louis, MO). Chitosan (molecular weight 100,000 – 300,000 Da) was purchased from Acros Organics (Fair Lawn, NJ). Sodium alginate (medium viscosity) was purchased from MP Biomedicals (Solon, OH). Soyabean L-α-Lecithin (98% phosphotidyl choline) was purchased from Calbiochem (San Diego, CA). Poloxamer 188 (Pluronic F68; Polyethylene- Polypropylene Glycol, block copolymer of ethylene oxide and propylene oxide, average molecular weight 8400 Da) was purchased from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ). Dimethyl sulfoxide (DMSO) was purchased from ThermoScientific Co. (Rockford, IL). Glacial acetic acid was purchased from Fisher Scientific (Fair Lawn, NJ). Eagle’s minimal essential cell culture medium (EMEM) was purchased from ATCC Co. (Manassas, VA). All chemicals utilized for preparing buffers are of the analytical grade. All materials were used as received without any further treatment.
Preparation of plain and celecoxib-loaded nanoparticles
To ensure sterility of our formulations, all tools used for preparation were sterile and all procedures were done under aseptic conditions to avoid any contamination. Also all the final formulations contained 0.01% w/v benzalkonium chloride as an anti-microbial agent. To maintain formulation isotonicity, all ophthalmic dosage forms including eye drops, in situ gelling systems and preformed gels were prepared using isotonic PBS as a vehicle.
Plain NPs were prepared using a spontaneous emulsification solvent diffusion technique [27,28] with some modifications as described in our published method [29]. Table 1 and 2 list the formulation ingredients used in the preparation of NPs in our optimization studies. Briefly, CS or ALG, at concentrations ranging from 0.1 to 0.5% w/v, was dissolved in the aqueous phase using a magnetic stirrer at 600 rpm. CS was dissolved in a minimal amount of 1% acetic acid solution and brought to volume with deionized water (DIW), while ALG was dissolved in DIW alone. Poloxamer 188 or PVA, both NP stabilizers, was dissolved in the polymer solution at a concentration ranging from 0.2 to 1.5% w/v. The pH of the CS solutions was adjusted to 4.5 using 0.1N NaOH. Lecithin was dissolved in dichloromethane (DCM) at a concentration ranging from 0.0 to 2% w/v, prior to adding acetone to the lecithin organic solution. The organic solution was injected into the aqueous solution (with magnetic stirring, 900 rpm) at an injection flow rate of 0.8 ml/min using an infusion pump (Fisher Scientific, Fair lawn, NJ). The emulsion was sonicated (Misonix S-4000, Qsonica LLC, Newtown, CT) at 100% amplitude for 10 min in an ice bath followed by stirring overnight at 200 rpm to allow complete evaporation of DCM. The NP dispersion was then filtered through a 1 μm Puradisc syringe filter (GE Healthcare, Buckinghamshire, UK) to remove any NP agglomerates. Fifty milliliters of DIW was added to the filtrate to facilitate diffusion of acetone to the aqueous phase. The NP suspension was centrifuged for 2 h at 60,000 rpm (SORVALL, WX Ultra Series Centrifuge, USA) and the NP pellet was washed three times by resuspension in 20 ml of DIW and recentrifugation to remove excess emulsifiers and trace acetone. The final NP pellet was suspended in 10 ml DIW containing 0.5 gm of trehalose (i.e., 5% w/v) as a cryoprotectant. After maintenance overnight in at -80°C, NPs were lyophilized (Freezone Lyophilizer, Labconco Corporation, USA) for 2 days at -50°C and reduced pressure (0.002 mbar). The freeze-dried NPs were sealed and maintained at 4°C until subjected for further analysis. Celecoxib-loaded NPs was prepared by the same method and celecoxib was dissolved in the organic phase in a concentration of 0.05%.
Table 1 : Composition of CS-NPs and their characterization.
Table 2 : Composition of ALG-NPs and their characterization.
Optimization of NPs formulation
To optimize our formulation, we studied the effect of several formulation parameters on final particle size, polydispersity index (PDI) and zeta potential. The variables we tested include: the type of polymer and its concentration; the type of stabilizer and its concentration; and the concentration of emulsifier (lecithin). Our criteria for selecting the optimum formula for each polymer included a small and consistent particle size and a high zeta potential. These formulations were further evaluated with regard to the effect of acetone in the organic phase, using DCM as a control. The effect of various sonication times (i.e., 0, 5, 10, 15 min) and the effect of lyophilization on the particle size was studied by measuring it before and after lyophilization keeping all other parameters constant.
Characterization of plain and celecoxib-loaded NPs
Measurement of particle size and zeta potential
To measure particle size and zeta potential, NPs were diluted in DIW to 0.5 mg/ml. Particle size and its variance, known as PDI, was measured using photon correlation spectroscopy using a zetasizer (Nanoseries, nano-ZS, Malvern Instruments Limited, UK). The zeta potential of NPs was measured using the laser doppler velocimetry function of the zetasizer by placing the diluted sample in an electrophoretic cell until a potential of 150 mV was established. Statistical analyses of variations in particle size and zeta potential data were performed using an unpaired t-test GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). All experiments were performed in triplicate.
Examination of the nanoparticles morphology
The morphology of the NPs was examined with a transmission electron microscope (TEM) (JEM-2000EX II Electron Microscope, JEOL, LTD, Tokyo, Japan). Nanoparticle suspensions were diluted with DIW to yield a concentration of 0.05 mg/ml and 2 μl of each suspension was placed on a 400 mesh copper grid covered with formvar (Electron Microscopy Sciences EMS, Hatfield, PA, USA). Grids were maintained in a desiccator until they were completely dry after which time they were examined by TEM.
Drug loading assessment
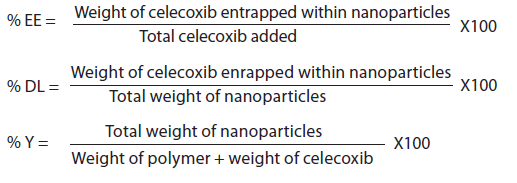
To determine amount of celecoxib incorporated into the NPs, five milligrams of celecoxibloaded NPs were weighed and dissolved in 2 ml DMSO in a 25 ml volumetric measuring flask and brought up to volume by absolute ethyl alcohol. The solution was assayed with a validated spectrophotometric method (-Quant universal microplate spectrophotometer Bio-Tek Instruments, Inc., USA) at 253 nm. The total drug content of each formulation was calculated from the standard curve (with a linearity coefficient (r) = 0.999). After controlling for any absorbance due to blank NPs, all measurements fell within the calibration linearity range of the standard calibration curve. Each experiment was repeated three times. The encapsulation efficiency (% EE), drug loading (% DL) and NP yield (% Y) were calculated using the following equations.
Preparation of plain ophthalmic dosage forms
The plain ophthalmic dosage forms that were evaluated are eye drops, a temperaturetriggered in situ gelling system, and a preformed gel. All formulations contained 0.01% w/v benzalkonium chloride as an anti-microbial. Eye drops were prepared by dissolving 2% w/v HPMC in phosphate buffered saline (PBS, 10 mM, pH 7.4). The in situ gelling system was prepared by dissolving 3% w/v HPMC in PBS then the solution is cooled to 4°C and 17% w/v poloxamer 188 was dissolved in such HPMC solution. The preformed eye gel was prepared by dissolving 4.5% w/v MC in PBS and allowing it to stand for 48 h with periodic stirring until a clear gel was formed. Chemicals used for the preparation of plain ophthalmic formulations and their ratios were selected after preliminary experiments to determine the optimal conditions that gave clear and transparent formulation with suitable consistency. The composition of the in situ gelling system was optimized after testing several prepared in situ gelling system formulations by dropping them in an artificial tears solution at 35°C ± 0.5 and observing the clarity of the formed gel, gelation time and the time taken for the formed gel to dissolve [30].
Preparation of ophthalmic formulation containing celecoxib-loaded NPs
Celecoxib-loaded NPs were incorporated in the above mentioned plain ophthalmic dosage forms to give a final concentration of celecoxib equivalent to 0.1% w/v. Each formulation was mixed well using magnetic stirrer (600 rpm) to ensure homogenous NP distribution. Subsequently each formulation was placed in a clean, dry and sterile glass container and maintained at 4°C until subjected to further analyses.
Evaluation of ophthalmic formulations containing celecoxib-loaded NPs
Measurement of pH
One gram of each formulation was dispersed in 20 ml DIW. The pH was determined using a pH meter (Corning pH meter 440, Corning Incorporated, Corning, NY).
Determination of formulation viscosity
Viscosities were determined using a cone (1.5°) and plate rotary viscometer (Brookfield DVII+ programmable viscometer, Brookfield Engineering Laboratories INC., USA). Five hundred microliters of each formulation was placed on the stationary plate of the viscometer and allowed to equilibrate for 5 min to reach the running temperature before each measurement. The measurements were performed at 35°C ± 0.5. To study temperatureinduced gelation, measurements were also performed at 25°C ± 0.5 using the in situ gelling systems. Measurements were performed at angular velocities ramping from 0 to 200 rpm (equivalent to shear rate 0 to 768 s-1) and from 200 to 0 rpm. Viscosity values were registered every 20 s. Averages of the two readings were used to calculate the viscosity of each formulation [30]. Viscosity values at 10 rpm were used for comparative purpose. The experiments were repeated three times and the results were calculated as mean ± SD.
Uniformity of formulation drug content
One gram of each formulation was placed in a stoppered volumetric 100 ml flask. Five milliliters of DMSO was added and each flask was shaken for 30 minutes. Subsequently, 50 ml of absolute ethyl alcohol was added and flask was shaken for additional 30 minutes after which it was brought to volume using absolute ethyl alcohol. The solution was centrifuged for 15 minutes at 10,000 rpm, after which time the suspension was passed through a syringe filter (pore size 0.22 μm). The clear solution was assayed spectrophotometrically at 253 nm for its drug content with appropriate negative controls. The experiments were repeated three times and the results were calculated as mean ± SD.
In vitro release study of celecoxib from ophthalmic formulations and kinetic evaluations
The release of celecoxib from different ophthalmic formulations containing drug-loaded NPs was performed using the Fast Micro-Equilibrium Dialyzer (Harvard Apparatus Co., Holliston, MA). The fast micro-equilibrium dialyzer consists of two chambers (i.e., donor chamber and acceptor chamber) each of 1500 μl capacity and 1.5 cm diameter, separated by semipermeable regenerated cellulose membrane (Harvard Apparatus Co., Holliston, MA) with molecular weight cut-off of 25,000 Da. One hundred milligrams of the ophthalmic formulations containing celecoxib-loaded NPs or the control preparation (i.e., 0.1% celecoxib suspension in PBS) were placed in the donor chamber in combination with 100 μl PBS. Warmed PBS (1.5 ml at 35°C ± 0.5) was placed in the acceptor chamber. The two chambers were tightly closed and the dialyzer was kept in thermostatically controlled shaker (35°C ± 0.5 and 50 rpm). The dialyzer was placed with the donor chamber was above and the acceptor chamber below. At predetermined time intervals ranging from 30 minutes to 24 hours, the entire medium in the acceptor chamber was withdrawn and replaced by 1.5 ml of fresh warmed PBS to ensure sink conditions. The released amount of celecoxib in the withdrawn samples from each formulation was analyzed spectrophotometrically at 253 nm to determine its drug content. Control experiments were performed using plain NPs incorporated in plain dosage form vehicles. The experiments were repeated three times and the concentrations were calculated from the standard curve as noted above. The release data were statistically analyzed using one-way ANOVA test followed by Tukey-Kramer multiple comparisons test [31]. Statistical calculations were carried out using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA).
In vitro evaluation of formulation cell toxicity
In vitro cytotoxicity of the formulations was evaluated with an MTT assay [32]. Briefly, HEK293 cells were seeded (4400 cells/well) into each well of 96-well plate (Costar 3596, Corning Inc., Corning, NY, USA) and allowed to incubate overnight in EMEM medium and humidified atmosphere (98% relative humidity, 5% CO2, 37°C). Two hundred microliters of the diluted formulations (i.e., 65 μl formulation + 135 μl of EMEM medium) was added to each well. This is equivalent to 65 μg celecoxib or 0.446 mg CS-NPs or 0.487 mg ALG-NPs. After a 24 h incubation, the formulation was replaced with 160 μl of growth medium. Forty microliters of MTT reagent (5 mg/ml) was added to each well. After 4 hrs of incubation at 37°C, the medium was replaced with 200 μl DMSO as a formazan solvent. The plate was then shaken for 15 min. Optical absorbance was measured at 570 nm with the use of a microplate reader (-Quant Universal Microplate Spectrophotometer, Bio-Tek Instruments, Inc.) and converted to percent cell viability relative to the control (untreated) cells. Statistical analysis of the percent cell viability data was performed using a one-way ANOVA with Tukey- Kramer multiple comparison tests [31]. Each experiment was done in six replicates.
Results and discussion
The goal of our investigation was to devise a non-invasive sustained release topical ophthalmic celecoxib formulation to overcome the drawbacks of systemic dosage. To achieve this goal, we optimized the preparation of two NPs synthesized from natural polymers and three delivery formulations.
Characterization and optimization of plain nanoparticles
Cationic CS-NPs and anionic ALG-NPs were prepared using a spontaneous emulsification solvent diffusion method. To optimize the NP formulations, the effect of various formulation parameters on the particle size and zeta potential were studied. The method of production is very simple, as the production of nano-sized particles required only simple magnetic stirring followed by sonication without successive mechanical treatment. The optimized NPs formulations that were chosen for drug incorporation and preparation of our ophthalmic formulation were selected because they had the lowest particle size and PDI – which would allow them to pass through biological barriers – and the highest zeta potential to achieve the highest stability in aqueous systems.
Effect of polymer and its concentration
Increasing the polymer concentration of either CS or ALG led to an increase in the viscosity of the preparation medium, which in turn increased the particle size and the size distribution of the resultant NPs (Table 1 and 2, Formulations 1-8). It is possible that the increased viscosity of the preparation medium caused enlarged organic phase droplets to be dispersed in the aqueous phase. This process may then have interfered with the interfacial hydrodynamic phenomenon that is responsible for spontaneous emulsification process [33]. Moreover, at the same concentration, CS-NPs had a larger particle size than ALG-NPs. This may be due to the higher viscosity of the CS solution than ALG solution because of the higher molecular weight of CS used. CS-NPs also possessed a positive charge due to the presence of amine groups on its surface. This positive charge was enhanced by increasing the polymer concentration. The negative charge of the ALG-NPs is due to the presence of the free carboxylic groups on its surface. Like CS-NPs, the charge of the ALG-NPs was enhanced by increasing the polymer concentration used to produce the NPs.
Effect of stabilizer and its concentration
Increasing the stabilizer concentration with either Poloxamer 188 or PVA decreased the particle size of the NPs and improved its size distribution (Table 1 and 2, Formulations 2, 6 and 9-14). This may be due to the fact that the presence of the stabilizer in the external aqueous phase stabilized the emulsion droplets against coalescence. This stabilization effect became more dominant at higher stabilizer concentrations, which decrease the size and PDI of NPs. These results are in agreement with those obtained by Mao et al., who reported that increasing PVA concentration caused a marked decrease in the particle size and PDI of PLGA microspheres loaded with fluorescein isothiocyanatelabeled dextrans [34]. The stabilizer concentration also had an inverse relationship with the zeta potential, which could be attributed to the shielding effect. This property is due to stabilizer that is incorporated in the thin film on the surface of the NPs, which may partially mask the surface charge. These results are in agreement with those obtained by Sahoo et al., who demonstrated that increasing PVA concentration from 0.5% to 5% led to a decrease in the negative zeta potential of PLGA-NPs due to the increase in the residual amount of PVA on the particle surface which acted as a shield that mask the negative charge of PLGA-NPs [35]. Also Redhead et al., reported a similar reduction in the negative zeta potential of PLGA-NPs after coating with Poloxamer 407 and Poloxamine 908, which they attributed to the shielding effect exerted by the stabilizers [36]. We found that the zeta potential of NPs prepared with PVA were slightly lower than that of Poloxamer 188, suggesting that the shielding effect of PVA was greater than that of poloxamer 188. This may be because PVA was more strongly attached to the NPs surface than Poloxamer, which made it more difficult to remove during washing resulting in a stronger shielding effect and net higher surface coverage [37].
Effect of lecithin and its concentration
The particle size data in Table 1 (Formulations 2 and 15-19) and Table 2 (Formulations 6 and 15-19) demonstrated that lecithin is necessary for NP formation because without lecithin (Formulation 15 in both Table 1and 2), the preparation medium was very viscous gel that inhibited NP formation. In fact, we observed that the effect of lecithin on the particle size of NPs is a biphasic process. Specifically, increasing lecithin concentration from 0.0% to 1% led to a decrease in the particle size for both CS-NPs and ALG-NPs, but a further increase in lecithin concentration above 1% led to increase in NPs particle size. Increasing lecithin concentration from 0.0% to 1% led to an increase in its emulsification capacity that encourage the spontaneous emulsification process yielding smaller particle size. Increasing the lecithin ratio above 1% (i.e. optimal emulsifier concentration at which saturated emulsifier packing located at the surface of NPs) may have led to deposition of more lecithin in the interfacial film formed on o/w interface during emulsification which in turn led to an increase in the size of NPs. The lecithin concentration had an inverse relationship with the positive zeta potential values of CS-NPs, and a direct relation with the negative zeta potential of the ALG-NPs. As previously stated, the higher the lecithin concentration, the higher the lecithin content in the thin film located on the surface of NPs. Since lecithin carried a negative charge, the positive charges of CS-NPs were partially neutralized by the negative charge of lecithin. Thus, increasing lecithin concentration led to a decrease in the positive zeta potential of CS-NPs, while increasing the negative one of ALG-NPs [38].
Effect of acetone in the organic phase
The data presented inTable 3 demonstrate that acetone is a very important factor to produce smaller NPs. As shown in the table, NPs prepared without acetone have a larger particle size (552 and 395 nm for CS-NPs and ALG-NPs, respectively) compared to (107 and 138 nm for CSNPs and ALG-NPs, respectively) when using acetone. The presence of acetone in the organic phase facilitates the formation of smaller NPs without successive mechanical treatment. Because acetone is miscible with both DCM and water, it decreases the interfacial tension during the emulsification process resulting in a smaller particle size. In addition, the turbulence of the interface caused by the rapid acetone diffusion from the organic phase to the aqueous phase increases the area of the interface due to the spontaneous division of organic droplets into smaller ones. These smaller droplets still contain acetone that undergoes additional diffusion to the surrounding aqueous phase, thus producing additional interface turbulence. This step was repeated many times in our investigation, resulting in the formation of smaller NPs each time. These results agree with those obtained by Niwa et al., who reported that the presence of acetone in the organic phase is an essential factor for forming nano-sized spheres [27]. Also, El-Shabouri reported that acetone is very important in producing particles in the nano-size range, while its absence produced particles with average diameter greater than 1.2 μm [28]. In this investigation, there were significant decreases in zeta potential of both cationic and anionic NPs prepared in absence of acetone when compared with those prepared in the presence of acetone (p < 0.05) this may be due to changing in the arrangement of the surfactants molecules on the NPs surface as a result of absence of acetone.
Effect of sonication time
Sonication is very important variable in the production of NPs of small size with a narrow size distribution, as exerting energy is a fundamental step in emulsification process. Our results presented in Table 3 show that without sonication, the NPs were of a larger particle size (682 and 485 nm for CSNPs and ALG-NPs, respectively) with high PDI values (0.971 and 0.724 for CSNPs and ALG-NPs, respectively). Increasing sonication time from 0 to 10 min led to a significant decrease in both particle size and PDI (p < 0.0001). These results are in agreement with those obtained by Mainardes and Evangelista, who reported that increasing the sonication time from 1 to 20 min greatly decreased the particle size and improved the size distribution of praziquantel-loaded PLGA-NPs [39]. However, increasing the sonication time to 15 min had a reverse effect as the particle size was slightly increased. This may be due to the aggregation of very small particles that resulted from prolonged sonication time to form larger ones. These results are in accordance with those observed by Tripathi el al., who reported that 20 min is the ideal sonication time for their formulation and sonication for 24 min increased the particle size of rifampicin-loaded PLGA-NPs [40].
Table 3 : Effect of different formulation parameters on particle size, PDI and zeta potential of optimized formulae.
Effect of lyophilization
Lyophilization is performed to remove water from the formulation and therefore improve NP stability upon storage. The process of freeze-drying is stressful and hence a cryoprotectant is added in the process in order to maintain the particle size and help NP reconstitution [41]. Our data presented in Table 3 demonstrate that the lyophilization process has no significant effect on the particle size or PDI of NPs made from either CS or ALG (p > 0.05). This indicated the cryoprotective efficiency of trehalose when used in a concentration of 5% to maintain the average particle size of NPs during freeze-drying process. Similar observations were reported by Sonaje et al., [42] and Hafner et al., [43]. Regarding NPs zeta potential, it was found that there was a minor decrease in zeta potential values after lyophilization for both positively charged CSNPs and negatively charged ALG-NPs. This may be explained by the NP surface masking the effect contributed by the cryoprotectant due to the hydrogen bonding between the (OH) groups of trehalose and NPs surface or due to the rearrangement of the surfactants (lecithin, PVA and Poloxamer 188) molecules on the NPs surface during freeze drying process. These results are in accordance with those obtained by De Chasteigner et al., who reported that there is a considerable decrease in the negative zeta potential of itraconazole-loaded poly (∑-caprolactone) NPs after freeze drying using 10% sucrose as cryoprotectant [44].
Characterization of celecoxib-loaded nanoparticles Particle size and zeta potential
Our data presented in Table 4 demonstrate that there was a non-significant increase in the mean particle size of CS-NPs (p > 0.05) and a significant increase in that of ALG-NPs after incorporation of celecoxib, compared to the mean particle size of the plain NPs (p < 0.05) without any significant effect on the NPs zeta potential (p > 0.05). Although there was an increase in the particle size of the drug loaded NPs, its size is still in the reasonable range required for an accepted in vivo study as it is known that NPs less than 200 nm are considered acceptable for passive drug targeting [45,46].
Table 4 : Characteristics of optimized celecoxib-loaded NPs.
Celecoxib encapsulation efficiency
Our results (Table 4) demonstrate that the encapsulation efficiency of celecoxib was very high (89.88 and 75.38% for CS-NPs and ALG-NPs, respectively) with a final drug loading of 14.57 and 13.34% for CS-NPs and ALG-NPs, respectively. Since, celecoxib is a very poorly water soluble drug (the measured celecoxib solubility in deionized water is 6.98 ± 0.021 μg/ml at 25°C ± 0.5), so it was preferentially partitioned in the organic phase during spontaneous emulsification step (the measured celecoxib partition coefficient in n-octanol/water system is 3.418 ± 0.082 at 25°C ± 0.5) and consequently, only a small amount of celecoxib was lost in the aqueous phase. Also, the small particle size achieved in our optimized formulations greatly improved the celecoxib encapsulation efficiency. The use of acetone, which is a highly diffusible and volatile solvent, also had a positive effect on increasing celecoxib encapsulation efficiency. Upon organic phase injection into the aqueous phase during NPs preparation, acetone was removed rapidly leaving the poorly water-soluble drug within NPs away from the external aqueous phase. These results are in accordance with those obtained by Kim et al., who reported that there was an improvement in the encapsulation efficiency of celecoxib in PLGA NPs when using acetone as a solvent compared to other solvents such as dimethylformamide, dimethylsulfoxide, 1,4-dioxane and dimethylacetamide [47].
Nanoparticle surface morphology
The TEM images (Figure 1) of both plain and celecoxibloaded CS-NPs and ALG-NPs supports the particle size data obtained by the zetasizer. Morphologically, the NPs have a distinct spherical shape with a solid dense polymer core surrounded by evenly distributed coat that may be comprised of lecithin and NPs stabilizer (PVA or Poloxamer 188). Drug incorporation did not affect the shape of the NPs.
Figure 1 : TEM image of plain CS-NPs (A), TEM image of celecoxib-loaded CS-NP (B), size and zeta potential distribution curves for celecoxib-loaded CS-NPs (C), TEM image of plain ALG-NP (D), TEM image of celecoxib-loaded ALG-NP (E), size and zeta potential distribution curves for celecoxib-loaded ALG-NPs (F).
Characterization of formulations containing celecoxib-loaded NPs
Characterization of ophthalmic formulations (i.e., eye drops, in situ gelling system and preformed gel) containing either celecoxib-loaded CS-NPs or ALG-NPs were evaluated as follows:
pH of the formulations
The pH of eye tears is 7.4 and due to its natural buffering capacity, it can tolerate ophthalmic formulations in a wide pH range from 3.5 to 8.5. Moreover, the typical ophthalmic dose is 1 or 2 drops, which can be easily accommodated by the eye and is rapidly restored to its normal pH value by the intrinsic buffering system [48]. Our data presented in Table 5 show that the prepared ophthalmic formulations have pH values ranging from 7.50 to 7.67, which can be easily tolerated by the eye without any irritation or discomfort.
Table 5 : Characteristics of ophthalmic formulations containing optimized celecoxib-loaded NPs.
Formulations viscosity
Figure 2 shows the rheological profiles of the prepared ophthalmic formulations containing either CS-NPs or ALG-NPs. The rheological profiles of eye drops (Figure 2A) showed that the eye drops formulations possessed Newtonian flow behavior, as they exhibited constant viscosities at various shear rates or angular velocities. These results are in agreement with those obtained by Wu et al., who observed that formulations containing HPMC alone exhibited Newtonian flow behavior under both physiological and non-physiological conditions [49]. While the rheological profiles of in situ gelling systems and preformed gels (Figures 2B and 2C) possessed non- Newtonian pseudoplastic (i.e. shear thinning) flow behaviors, as they exhibited high viscosities at lower shear rates and the viscosity decreased as the shear rate increased.
Figure 2 : Rheological profiles of celecoxib-loaded CS-NPs and ALG-NPs eye drops at 35°C (A), in situ gelling systems at 25 and 35°C (B) and preformed gels at 35°C (C).
This pseudoplastic behavior is preferred for topical ophthalmic preparations for two reasons. The first is to not interfere with the pseudoplastic properties of the precorneal tear film and the second is to not cause patient discomfort during eye blinking process. While the ocular shear rate is very low (0.03 s-1) during inter-blinking rest periods, it is very high during blinking (4250-28500 s-1) [3]. Therefore an ideal formulation would have a higher viscosity that prevents its drainage from the eye during inter-blinking periods and a lower viscosity during blinking that would not cause patient discomfort. Our formulations fulfill these criteria. They are also in accordance with studies by Hammer and Burch, who found that MC formulations possessed pseudoplastic properties [50] and Lin and Sung, who found that formulations containing poloxamer possessed pseudoplastic behaviors [51]. Temperatureinduced gelation of the in situ gelling systems, as seen in Figure 2B, demonstrate that formulation viscosity is increased by increasing the temperature of the measurement from a non-physiological temperature (25°C) to a physiological temperature (35°C), which suggests that our in situ gelling system will undergo sol-gel transition upon instillation into the eye.
Drug content
Table 5 illustrates that the actual celecoxib content of the prepared ophthalmic formulations ranged from 98.33 to 102.11% of that loaded into celecoxib-loaded NPs. These result showed that the deviation of the drug contents from the originally added active constituents are less than ± 5%, which complies with the USP official requirement [49]. Also the small values of the standard deviation indicate a uniform distribution of the celecoxib-loaded NPs within the ophthalmic formulations vehicles.
In vitro release of celecoxib and its kinetics
In vitro release studies were conducted for only 24 h, as the tested formulations were intended for topical ophthalmic use. Blank formulations did not have any significant absorbance at 253 nm. The release profiles as shown in Figure 3 confirmed that it is possible to prepare sustainedrelease topical ophthalmic formulations containing either celecoxib-loaded bioadhesive positively charged CSNPs or negatively charged ALG-NPs. All the formulations possessed a sustained drug release rate that was free from any burst release that may cause toxicity. Burst release is a characteristic of drug released from monolithic NP systems due to the rapid dissolution of the drug molecules located on or near the NP surface into the surrounding fluid. In our study, the absence of burst release may be due to two factors. The first is that the NPs are not suspended directly in the dissolution medium; rather they are dispersed in its dosage form vehicles (i.e., eye drops, in situ gelling system or gel). Therefore, once the drug is released from the NPs, it dispersed in the dosage form prior to being released to the medium. This step-wise sequence sustained the release of drug and decreased the burst effect. The second factor is the presence of a coat around the NPs that was confirmed by TEM (Figure 1). This coat shielded the NP surface and prevented the rapid burst release upon dilution with the release medium. In the literature, many studies coat NPs using certain coating materials to decrease or prevent the burst release. Huang et al., reported that coating of NPs with gelatin reduced the initial burst release of propranolol HCl and lidocaine from PLA/PEG NPs [52]. Also, Garcia-Fuentes et al., reported that coating of lipid NPs with chitosan inhibit the burst release of salmon calcitonin [53]. Budhian et al.,showed that chitosan coating of PLGA-NPs reduced the burst release of haloperidol [54].
Figure 3 : Celecoxib release profiles from ophthalmic formulations containing celecoxib-loaded CS-NPs and ALG-NPs.
The percentage of the cumulative amount of celecoxib released after 24 h from CS-NPs preparations was 61.5, 50.1 and 44.6% for eye drops, preformed gel and the in situ gelling system, respectively. From ALG-NPs preparations, the cumulative release was 51.3, 46.7 and 40.1% for eye drops, preformed gel and in situ gelling system, respectively. The release of celecoxib from all preparations was significantly different from that of the control preparation (p < 0.001), as the control preparation showed an irregular uncontrolled release pattern that is only governed by the low celecoxib solubility in the release medium (the measured celecoxib solubility in PBS is 6.18 ± 0.043 μg/ml at 25°C ± 0.5). The celecoxib release profiles from CSNPs preparations were significantly different from each other (p < 0.01). Similarly, for ALGNPs preparations, the celecoxib release profiles were also statistically different from each other (p < 0.05). CS-NP preparations of like kind showed significantly higher celecoxib release profiles than ALG-NPs formulations (p < 0.05), These higher release rates of the CS preparations may be due to the smaller particle size of CS-NPs, as the smaller the particle size the higher the surface area available for drug release and thus the higher the release rate [55]. Also, the dosage form vehicles played an important role in controlling the celecoxib release rate, as the release rates of the formulations were in the following order: eye drops > preformed gel > in situ gelling system. This may be due to the difference in their viscosities upon dilution with PBS at 35°C, as the higher the viscosity, the slower the release rate [56].
In order to determine the kinetic model that best describes the release mechanism, the in vitro release data were analyzed according to zero-order, first-order and Higuchi diffusion models. The model with the highest correlation coefficient was selected as the best fit [57]. We used the Korsmeyer-Peppas semi-empirical model for additional in depth analysis [58], where the value of the release exponent is determined by the release mechanism and thus can be used to characterize it [59].
Results of release kinetic analyses (Table 6) showed that celecoxib released from all the formulations followed the Higuchi model, suggesting that the release mechanism is diffusion. In contrast, the control preparation followed zero-order kinetics, that suggesting dissolution of celecoxib from its crystals. Further analysis of the release data using the Korsmeyer-Peppas equation showed that the release exponents (n) for all formulation ranged from 0.45 to 0.89, which indicated that they exhibited non-Fickian or anomalous diffusion. Therefore, the drug release from the formulation was not by a pure diffusion mechanism, but was rather a mixture of both diffusion from the NPs and erosion of the polymers. The value of (n) for the control preparation was 0.9711, which indicates a super case transport II suggesting that the release is governed only by erosion of celecoxib crystals during its dissolution [59].
Table 6 : In vitro release kinetics of celecoxib.
In vitro cytotoxicity
To evaluate possible cell toxicity of the formulations, we used a volume of 65 μl, which is the highest estimated value for an ophthalmic dose, as the volume of a single drop obtained from multi-dose eye drops bottles was reported to range from 33.8 to 63.4 μl [60]. The HEK293 cell line was selected to test the in vitro cytotoxicity of our formulations due to its high sensitivity toward toxic materials. This characteristic has made this cell line widely used by a large number of researchers to estimate the in vitro cell toxicity of their pharmaceutical preparations before in vivo testing [32,61–64]. Figure 4 shows that all tested formulations – plain dosage form vehicles, plain NPs, celecoxib suspension, CS-NPs formulations and ALGNPs formulations – were not toxic, as the cell viability of all formulations ranged from 89.9 to 97.7%. These results showed no statistically significant differences (p > 0.05) compared to the control preparation containing 0.1% celecoxib suspension. Therefore, the minimal toxicity that we measure may be due to the drug itself, rather than the formulations. Also the percent cell viability of all formulations showed high statistically significant differences (p < 0.001) compared to the positive control containing 1% of the cytotoxic agent Triton-X 100. This may be due to the fact that all the polymers used in our formulations either for preparation of the vehicles [65] or the NPs [1,9] possessed an excellent biocompatibility.
Figure 4 : Cytotoxicity histograms of ophthalmic formulations containing celecoxib-loaded CS-NPs and ALG-NPs.
Conclusions
This paper describes the preparation and evaluation of new topical ophthalmic sustainedrelease dosage forms containing celecoxib-loaded CS or ALG NPs prepared by a spontaneous emulsification solvent diffusion method. The optimized NP formulations had desirable particle sizes, zeta potentials and surface morphology. The prepared formulations possessed pH and viscosity values that are compatible with the eye and have uniform drug contents that complied with the USP official requirement. In vitro release data of all ophthalmic formulations showed a sustained release, free of any burst effect; the release profiles follow a Higuchi non-fickian diffusion mechanism. The in vitro cytotoxicity results revealed that all prepared formulations were non-toxic. Statistical analysis of cytotoxicity results showed that there were no statistically significant differences between the formulations and the control preparation that contained 0.1% celecoxib suspension. Although both CS- and ALG-NPs possessed bioadhesive properties, we suggest that CS-NP preparations will be more efficacious than ALG-NPs due to the positive charge carried by CS. This feature improves its adherence to the negatively charged eye surface, resulting in longer ocular contact time and therefore more sustained effect. Because these formulations are intended for topical use, CS is preferred over ALG due to its penetration enhancing properties [10]. In vivo studies and accelerated stability studies of the ophthalmic formulations stored at three different temperatures (30, 35 and 45°C) for six months are in progress. Our investigation has determined the optimal formulations for sustained delivery of celecoxib to treat conditions of the eye.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MMJ is the corresponding author who contributed to the overall study design, as well as drafting, organizing, and revising the manuscript. MMI was solely responsible for the practical work of this study. Further, he performed the statistical analysis of data and he has been involved in drafting, organizing the manuscript. AHA and OAS have a contribution toward the conception and design of the study. All authors have read and approved this version of the final manuscript.
Acknowledgements and funding
The authors thank Drs. Shankar Swaminathan and Mallika Palamoor for their critical reading of this manuscript. They also thank Dr. Joel Bumgardner for the use of his viscometer. This study was supported by The Egyptian Government Joint Supervision Progam, The University of Tennessee Research Foundation and an Unrestricted Grant from Research to Prevent Blindness, New York, NY.
Publication history
Received: 29-Nov-2012 Revised: 14-Dec-2012
Re-Revised: 6-Jan-2013 Accepted: 23-Jan-2013
Published: 11-Feb-2013
References
- De S and Robinson D: Polymer relationships during preparation of chitosan-alginate and poly-l-lysine-alginate nanospheres. J Control Release 2003, 89:101-12. | Article | PubMed
- Alonso MJ: Nanomedicines for overcoming biological barriers. Biomed Pharmacother 2004, 58:168-72. |Article | PubMed
- Bourlais CL, Acar L, Zia H, Sado PA, Needham T and Leverge R: Ophthalmic drug delivery systems–recent advances. Prog Retin Eye Res 1998, 17:33-58. | Article | PubMed
- Smolin G, Okumoto M, Feiler S and Condon D: Idoxuridine-liposome therapy for herpes simplex keratitis. Am J Ophthalmol 1981, 91:220-5. | Article | PubMed
- Losa C, Calvo P, Castro E, Vila-Jato JL and Alonso MJ: Improvement of ocular penetration of amikacin sulphate by association to poly(butylcyanoacrylate) nanoparticles. J Pharm Pharmacol 1991, 43:548-52. | Article | PubMed
- Losa C, Marchal-Heussler L, Orallo F, Vila Jato JL and Alonso MJ: Design of new formulations for topical ocular administration: polymeric nanocapsules containing metipranolol. Pharm Res 1993, 10:80-7. |Article | PubMed
- Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ and Khar RK: Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm 2008, 68:513-25. | Article | PubMed
- Shahidi F and Abuzaytoun R: Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nutr Res 2005, 49:93-135. | Article | PubMed
- Alonso MJ and Sanchez A: The potential of chitosan in ocular drug delivery. J Pharm Pharmacol 2003,55:1451-63. | Article | PubMed
- De la Fuente M, Ravina M, Paolicelli P, Sanchez A, Seijo B and Alonso MJ: Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev 2010, 62:100-17. |Article | PubMed
- Varum KM, Myhr MM, Hjerde RJ and Smidsrod O: In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr Res 1997, 299:99-101. | Article | PubMed
- Temel A, Kazokoglu H and Taga Y: Tear lysozyme levels in contact lens wearers. Ann Ophthalmol 1991,23:191-4. | PubMed
- Rabea EI, Badawy ME, Stevens CV, Smagghe G and Steurbaut W: Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003, 4:1457-65. | Article | PubMed
- De Campos AM, Sanchez A and Alonso MJ: Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm 2001,224:159-68. | Article | PubMed
- Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F and Couvreur P: Development of a new drug carrier made from alginate. J Pharm Sci 1993, 82:912-7. | Article | PubMed
- Bodmeier R and Wang J: Microencapsulation of drugs with aqueous colloidal polymer dispersions. J Pharm Sci 1993, 82:191-4. | PubMed Sechoy O, Tissie G, Sebastian C, Maurin F, Driot JY and Trinquand C:A new long acting ophthalmic formulation of carteolol containing alginic acid. Int J Pharm 2000,207:109-16. | Article | PubMed
- Schalnus R: Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica 2003,217:89-98. | Article | PubMed
- Sechoy O, Tissie G, Sebastian C, Maurin F, Driot JY, Trinquand C: A new long acting ophthalmic formulation of carteolol containing alginic acid. Int J Pharm 2000, 207:109-116 | Article |PubMed
- Kim SJ, Flach AJ and Jampol LM: Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol 2010, 55:108-33. | Article | PubMed
- Johnson EI, Dunlop ME and Larkins RG: Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Curr Eye Res 1999, 18:79-82. | Article | PubMed
- Tong CT, Howard SA, Shah HR, Van Quill KR, Lin ET, Grossniklaus HE and O’Brien JM: Effects of celecoxib in human retinoblastoma cell lines and in a transgenic murine model of retinoblastoma. Br J Ophthalmol 2005, 89:1217-20. | Article | PubMed Abstract | PubMed Full Text
- Bauer AM, Fiehn C and Becker MD: Celecoxib, a selective inhibitor of cyclooxygenase 2 for therapy of diffuse anterior scleritis. Am J Ophthalmol 2005, 139:1086-9. | Article | PubMed
- Ayalasomayajula SP and Kompella UB: Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res 2004, 21:1797-804. |Article | PubMed
- Amrite AC, Ayalasomayajula SP, Cheruvu NP and Kompella UB: Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci 2006, 47:1149-60. | Article | PubMed Abstract | PubMed Full Text
- Ayalasomayajula SP and Kompella UB: Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol 2005, 511:191-8. | Article | PubMed
- Cheruvu NP, Amrite AC and Kompella UB: Effect of eye pigmentation on transscleral drug delivery.Invest Ophthalmol Vis Sci 2008, 49:333-41. | Article | PubMed Abstract | PubMed Full Text
- Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y: Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D, L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. Journal of controlled release1993, 25:89-98. | Article
- El-Shabouri MH: Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int J Pharm 2002, 249:101-8. | Article | PubMed
- Ibrahim MM, Abd-Elgawad AE, Soliman OA and Jablonski MM: Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J Pharm Sci 2013. | Article | PubMed
- Liu Z, Li J, Nie S, Liu H, Ding P and Pan W: Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm 2006, 315:12-7. | Article | PubMed
- Kuzma JW, Bohnenblust SE: Basic Statistics for the Health Sciences. New York: NY: McGraw-Hill Education; 2005.
- Zhang Y and Zhuo RX: Synthesis, characterization, and in vitro 5-Fu release behavior of poly(2,2-dimethyltrimethylene carbonate)-poly(ethylene glycol)-poly(2,2-dimethyltrimethylene carbonate) nanoparticles. J Biomed Mater Res A 2006, 76:674-80. | Article | PubMed
- López-Montilla JC, Herrera-Morales PE, Pandey S, Shah DO: Spontaneous emulsification: mechanisms, physicochemical aspects, modeling, and applications. Journal of dispersion science and technology2002, 23:219-268. | Article
- Mao S, Xu J, Cai C, Germershaus O, Schaper A and Kissel T: Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm 2007, 334:137-48. | Article | PubMed
- Sahoo SK, Panyam J, Prabha S and Labhasetwar V: Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release 2002, 82:105-14. | Article | PubMed
- Redhead HM, Davis SS and Illum L: Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release 2001, 70:353-63. | Article | PubMed
- Scholes PD, Coombes AG, Illum L, Davis SS, Watts JF, Ustariz C, Vert M and Davies MC: Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J Control Release 1999, 59:261-78. | Article | PubMed
- Tan Q, Liu W, Guo C and Zhai G: Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomedicine 2011, 6:1621-30. | Article | PubMed Abstract |PubMed Full Text
- Mainardes RM and Evangelista RC: PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm 2005, 290:137-44. | Article | PubMed
- Tripathi A, Gupta R, Saraf SA: PLGA nanoparticles of anti tubercular drug: Drug loading and release studies of a water in-soluble drug. Int J PharmTech Res 2010, 2:2116-2123. | Pdf
- Yadav KS and Sawant KK: Modified nanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech 2010, 11:1456-65. | Article | PubMed Abstract | PubMed Full Text
- Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ and Sung HW: Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials 2010, 31:3384-94. | Article | PubMed Abstract | PubMed Full Text
- Hafner A, Durrigl M, Pepic I, Filipovic-Grcic J: Short- and long-term stability of lyophilised melatonin-loaded lecithin/chitosan nanoparticles. Chem Pharm Bull (Tokyo) 2011, 59:1117-1123. | Article
- De Chasteigner S, Cavé G, Fessi H, Devissaguet JP, Puisieux F: Freeze–drying of itraconazole–loaded nanosphere suspensions: a feasibility study. Drug development research 1996, 38:116-124. | Article
- Seijo B, Fattal E, Roblot-Treupel L, Couvreur P: Design of nanoparticles of less than 50 nm diameter: preparation, characterization and drug loading. International Journal of Pharmaceutics 1990, 62:1-7. |Article
- Scholes P, Coombes A, Illum L, Daviz S, Vert M, et al.: The preparation of sub-200 nmpoly (lactide-co-glycolide) microspheres for site-specific drug delivery. Journal of controlled release 1993, 25:145-153. |Article
- Kim TH, Jeong YI, Jin SG, Pei J, Jung TY, Moon KS, Kim IY, Kang SS and Jung S: Preparation of polylactide-co-glycolide nanoparticles incorporating celecoxib and their antitumor activity against brain tumor cells. Int J Nanomedicine 2011, 6:2621-31. | Article | PubMed Abstract | PubMed Full Text
- USP29-NF24. “The United States Pharmacopeia” 29th, The National formulary 24th: United States Pharmacopeial Convention Inc. MD; 2005.
- Wu H, Liu Z, Peng J, Li L, Li N, Li J and Pan H: Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int J Pharm 2011, 410:31-40. | Article |PubMed
- Hammer ME and Burch TG: Viscous corneal protection by sodium hyaluronate, chondroitin sulfate, and methylcellulose. Invest Ophthalmol Vis Sci 1984, 25:1329-32. | Article | PubMed
- Lin HR and Sung KC: Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release 2000, 69:379-88. | Article | PubMed
- Huang YY, Chung TW and Tzeng TW: A method using biodegradable polylactides/polyethylene glycol for drug release with reduced initial burst. Int J Pharm 1999, 182:93-100. | Article | PubMed
- Garcia-Fuentes M, Torres D and Alonso MJ: New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm 2005, 296:122-32. | Article | PubMed
- Budhian A, Siegel SJ and Winey KI: Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int J Pharm 2008, 346:151-9. | Article | PubMed
- Leroux JC, Allémann E, De Jaeghere F, Doelker E, Gurny R: Biodegradable nanoparticles—from sustained release formulations to improved site specific drug delivery. Journal of controlled release1996, 39:339-350. | Article
- Budai L, Hajdu M, Budai M, Grof P, Beni S, Noszal B, Klebovich I and Antal I: Gels and liposomes in optimized ocular drug delivery: studies on ciprofloxacin formulations. Int J Pharm 2007, 343:34-40. |Article | PubMed
- Tamizharasi S, Rathi JC and Rathi V: Formulation, and Evaluation of Pentoxifylline-Loaded Poly(epsilon-caprolactone) Microspheres. Indian J Pharm Sci 2008, 70:333-7. | Article | PubMed Abstract| PubMed Full Text
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA: Mechanisms of solute release from porous hydrophilic polymers. International Journal of Pharmaceutics 1983, 15:25-35. | Article
- Dash S, Murthy PN, Nath L and Chowdhury P: Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010, 67:217-23. | Pdf | PubMed
- German EJ, Hurst MA and Wood D: Reliability of drop size from multi-dose eye drop bottles: is it cause for concern? Eye (Lond) 1999, 13 ( Pt 1):93-100. | Article | PubMed
- Nogueira NP, Reis PA, Laranja GA, Pinto AC, Aiub CA, Felzenszwalb I, Paes MC, Bastos FF, Bastos VL, Sabino KC and Coelho MG: In vitro and in vivo toxicological evaluation of extract and fractions from Baccharis trimera with anti-inflammatory activity. J Ethnopharmacol 2011, 138:513-22. | Article | PubMed
- Liu Y, Wang T, He F, Liu Q, Zhang D, Xiang S, Su S and Zhang J: An efficient calcium phosphate nanoparticle-based nonviral vector for gene delivery. Int J Nanomedicine 2011, 6:721-7. | Article |PubMed Abstract | PubMed Full Text
- Caamal-Fuentes E, Torres-Tapia LW, Sima-Polanco P, Peraza-Sanchez SR and Moo-Puc R: Screening of plants used in Mayan traditional medicine to treat cancer-like symptoms. J Ethnopharmacol 2011,135:719-24. | Article | PubMed
- Staudacher I, Wang L, Wan X, Obers S, Wenzel W, Tristram F, Koschny R, Staudacher K, Kisselbach J, Koelsch P, Schweizer PA, Katus HA, Ficker E and Thomas D: hERG K+ channel-associated cardiac effects of the antidepressant drug desipramine. Naunyn Schmiedebergs Arch Pharmacol 2011, 383:119-39. | Article | PubMed
- Vandervoort J and Ludwig A: Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. Int J Pharm 2002, 238:77-92. | Article | PubMed