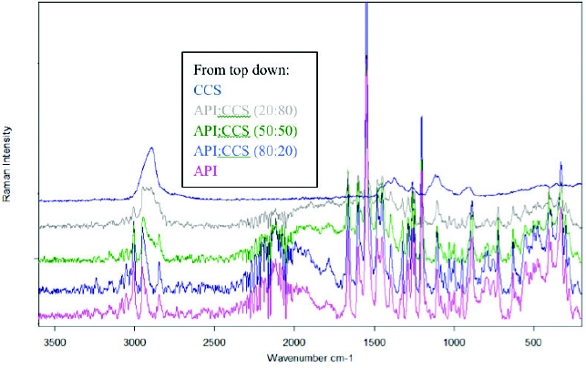
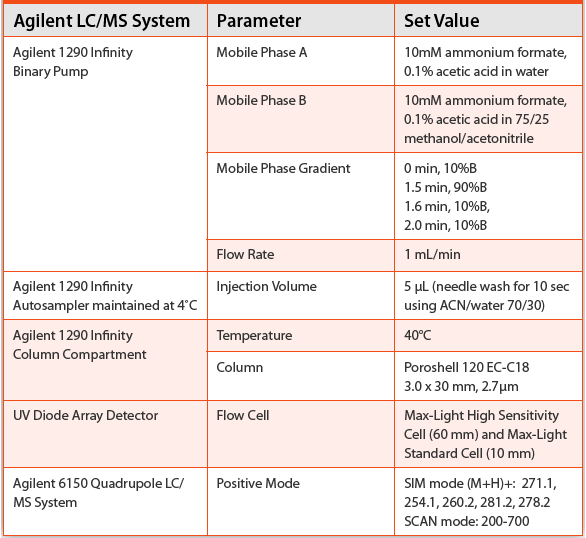
This entry was posted in Analytical Discussion and tagged biorelevant, class 2, Dissolution media, glibenclamide, glyburide.
The purpose of this studywas to predict the oral absorption of glyburide. Biorelevant dissolution
methods, combined with permeability measurements and computational simulations,
were used to predict the oral absorption of glyburide. The objectivewas to establish in vitro/in
vivo correlations (IVIVCs) based on the biopharmaceutics drug classification system. The solubility
of the glyburide powder was measured in different media. The dissolution behavior
of two commercial tablet formulations was tested in different media. Two chemical grades
of sodium taurocholate: low quality (LQ) = crude and high quality (HQ) = 97% purity, and egglecithin:
LQ = 60% and HQ= 99.1% purity were used to prepare fasted state small intestinal
fluid (FaSSIF). Simulated intestinal fluid (SIF) and blank FaSSIF without lecithin and taurocholate
(BL-FaSSIF) were used as controls. The dissolution tests were performed under
constant pH and dynamic pH conditions. The dynamic pH range from 5.0 to 7.5 simulated
the biological pH range of gastrointestinal (GI) tract in the fasted state. The drug permeability
was studied using Caco-2 cell line. The predictions of the fraction dose absorbed were
performed using GastroPlusTM. The results of the simulations were compared with actual
clinical data taken from a bioequivalence study. The solubility of glyburide was highest in
LQ-FaSSIF. The two tablet formulations had significantly different dissolution behaviors in
LQ-FaSSIF. The in vitro data was used as the input function into a simulation software. The
dynamic LQ-FaSSIF dissolution data achieved the best prediction of the average AUC and
Cmax of the clinically observed data. The present study shows that BCS based parameters
combined with software simulations can be used to establish an IVIVC for glyburide. In
vitro/in silico tools can potentially be used as surrogate for bioequivalence studies.