Integrated high capacity solid phase extraction-MS/MS system for pharmaceutical profiling in drug discovery

-
-
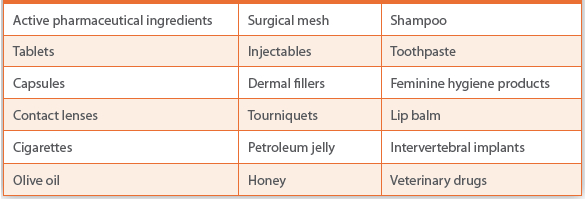
Analysis of Counterfeit FDA-Regulated Products at the Forensic Chemistry Center: Rapid Visual and Chemical Screening Procedures Inside and Outside of the Laboratory
Pharma Live, , Analytical Discussion, 0
Abstract Field and laboratory screening methods have been described for detecting counterfeit pharmaceutical packaging (cartons, bottles, etc) and finished...
-
Bio Analytical method development , validation & Transfer by using LC-MS/MS
Pharma Live, , Uncategorized, 0
About Authors:Satya Lakshmi.B*, P.Raju vel, Dr. P. Venkateswara Rao, Sindhu.G, Nikil Kumar.KA.M Reddy Memorial College of Pharmacy,Narasaraopet, AN University,...
-
Multifactor Non-linear Modeling for Accelerated Stability Analysis and Prediction – Courtesy (PharmTech)
Pharma Live, , Analytical Discussion, 0
Accelerated stability analysis is a strategy used to quickly evaluate alternative formulations, packaging, and processes. Accelerated linear studies are...
-
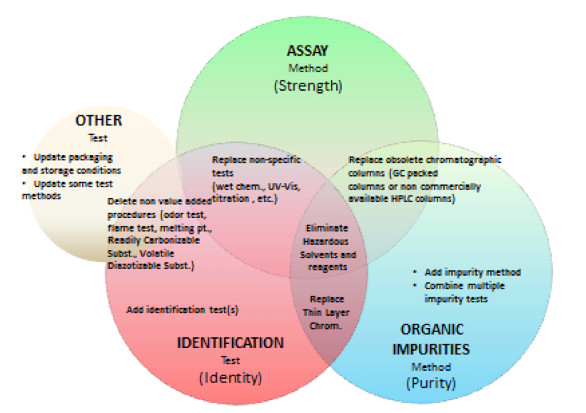
USP Monograph Modernization Initiative
Pharma Live, , Analytical Discussion, 0
Abstract The modernization of United States Pharmacopeia (USP) monographs continues to be a priority initiative. The traditional model has...
-
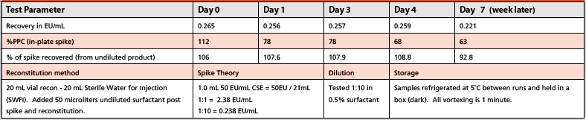
Endotoxin Test Concerns of Biologics
Pharma Live, , Analytical Discussion, 0
Limulus Amoebocyte Lysate (LAL) users are exploring regimens to study the effects of adding endotoxin to undiluted biologics in reaction to Chen’s...
-
Successful Sterility Test Failure Investigations—A Practical Approach
Pharma Live, , Analytical Discussion, 0
Introduction The intent of this article is to provide practical advice based upon gaps that the author has observed...
-
The Blood Brain Barrier and Drug Delivery to CNS
Pharma Live, , Analytical Discussion, 0
The blood brain barrier and drug delivery to cns